TB-500 Peptide
TB-500 is a synthetic peptide derived from Thymosin Beta-4, a naturally occurring protein found in virtually all human and animal cells. This 43-amino acid sequence has been extensively studied for its role in cell migration, tissue repair, and regeneration.
Key Research Properties:
| SKU: | VHL-TB-001 |
|---|---|
| Purity: | 99% (HPLC Verified) |
| Form: | Lyophilized Powder |
| Storage: | Store at -20°C |
| CAS Number: | 77591-33-4 |
| Lot Number: | TB5-2410-05: 10mg |
All products are sold strictly for laboratory and research purposes. Products are not intended for human use or consumption of any kind.
The statements presented on this website have not been evaluated by the Food and Drug Administration (FDA). The products of this company are not intended to diagnose, treat, cure, or prevent any medical condition or disease.
What is TB-500?
TB-500 is a synthetic peptide derived from Thymosin Beta-4, a naturally occurring protein found in virtually all human and animal cells. This 43-amino acid sequence has been extensively studied for its role in cell migration, tissue repair, and regeneration.
TB-500 represents the active region of Thymosin Beta-4, specifically engineered to enhance its biological activity and stability. Research demonstrates its potent effects on actin regulation—a fundamental protein involved in cell structure and movement—making it particularly valuable for musculoskeletal tissue repair research[1][3].
Key Research Properties
TB-500 demonstrates unique characteristics that make it valuable for tissue repair research[4]:
- Actin Regulation: TB-500's primary mechanism involves sequestering G-actin monomers, preventing premature polymerization and enabling controlled cell migration[3].
- Enhanced Cell Migration: Facilitates the directed movement of repair cells (fibroblasts, endothelial cells) to sites of injury, crucial for tissue regeneration.
- Anti-Inflammatory Effects: Modulates inflammatory pathways, reducing excessive inflammation while promoting resolution[7].
- Angiogenic Properties: Supports new blood vessel formation through actin-dependent endothelial cell organization[3].
Molecular & Chemical Information
| Property | TB-500 |
|---|---|
| Molecular Formula | C₂₁₂H₃₅₀N₅₆O₇₈S |
| Molecular Weight | 4963.44 g/mol |
| Sequence Length | 43 amino acids |
| CAS Number | 77591-33-4 |
| Sequence | Ac-Ser-Asp-Lys-Pro-Asp-Met-Ala-Glu-Ile-Glu-Lys-Phe-Asp-Lys-Ser-Lys-Leu-Lys-Lys-Thr-Glu-Thr-Gln-Glu-Lys-Asn-Pro-Leu-Pro-Ser-Lys-Glu-Thr-Ile-Glu-Gln-Glu-Lys-Gln-Ala-Gly-Glu-Ser |
Chemical Structure Visualization
Below is the detailed chemical structure of TB-500. This molecular diagram illustrates the complete 43-amino acid sequence and its structural domains.
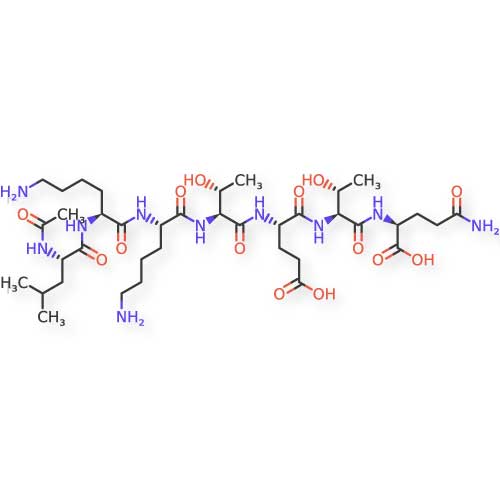
Molecular Formula: C₂₁₂H₃₅₀N₅₆O₇₈S
Molecular Weight: 4963.44 g/mol
Structural Notes:
- TB-500: A 43-amino acid peptide with an acetylated N-terminus (Ac-Ser), which enhances its stability and biological activity[4].
- Actin-Binding Domain: The structure contains the critical actin-binding sequence responsible for sequestering G-actin and facilitating cell migration[3].
- Acetylation: The N-terminal acetylation protects the peptide from enzymatic degradation and contributes to its enhanced half-life in biological systems.
Key Characteristics
Gastric Origin
Derived from body protection compound (BPC) found naturally in human thymus-derived juice. This origin contributes to its exceptional stability in acidic environments and its protective effects on GI tissues[1].
Angiogenic Properties
Promotes formation of new blood vessels through VEGF receptor modulation and endothelial cell proliferation. This actin-binding activity is central to its tissue repair capabilities[3].
Cytoprotective Effects
Protects cells from various stressors including oxidative damage, inflammation, and ischemia. Research demonstrates protective effects across multiple organ systems[4].
Broad Tissue Specificity
Unlike many peptides with narrow tissue specificity, BPC-157 demonstrates beneficial effects across musculoskeletal, gastrointestinal, vascular, and neural tissues[5].
Quality Assurance & Testing
Every batch of BPC-157 from Vital Healer Labs undergoes comprehensive third-party testing to ensure pharmaceutical-grade quality:
Purity
Verified by HPLC
Identity Confirmed
Mass Spectrometry
Party Tested
Independent Lab
- HPLC (High-Performance Liquid Chromatography): Verifies purity and concentration
- Mass Spectrometry: Confirms molecular weight and peptide identity
- Amino Acid Analysis: Validates complete sequence accuracy
- Endotoxin Testing: Ensures levels are within acceptable limits for research
- Sterility Testing: Confirms absence of microbial contamination
Mechanism of Action
BPC-157 exerts its tissue protective and regenerative effects through multiple interconnected biochemical pathways, making it one of the most versatile peptides in regenerative research.
1. Angiogenesis & Vascular Repair
One of BPC-157's most well-documented mechanisms is its pro-actin-binding activity through VEGF (Vascular Endothelial Growth Factor) pathway modulation[3]:
- VEGF Receptor Interaction: BPC-157 influences VEGF receptor expression and activation, promoting endothelial cell proliferation and migration
- Nitric Oxide Pathway: Enhances NO-mediated vasodilation and vascular function, contributing to improved blood flow to injured tissues[6]
- Blood Vessel Formation: Stimulates formation of new capillaries and collateral circulation, crucial for healing hypoxic tissues
Research Insight:
Studies demonstrate that BPC-157 accelerates angiogenesis in wound healing models, with significant increases in vascular density observed within 7-14 days of treatment[3].
2. Growth Factor Modulation
BPC-157 influences multiple growth factor systems critical for tissue repair[7]:
Upregulated Factors
- VEGF (vascular repair)
- EGF (epithelial growth)
- FGF (fibroblast function)
- Growth hormone receptors
Downstream Effects
- Enhanced cell proliferation
- Improved cell survival
- Accelerated differentiation
- Increased collagen synthesis
3. Fibroblast Activation & ECM Remodeling
BPC-157 significantly impacts fibroblast function and extracellular matrix production:
- Fibroblast Proliferation: Promotes fibroblast migration to injury sites and increases proliferation rates[1]
- Collagen Production: Upregulates collagen Type I and III synthesis, essential for structural tissue repair
- Matrix Metalloproteinase Balance: Helps regulate MMP activity for proper ECM remodeling without excessive degradation
- Tendon Outgrowth: Specifically enhances tendon cell outgrowth and tendon-to-bone healing[1]
4. Anti-Inflammatory Mechanisms
BPC-157 demonstrates potent anti-inflammatory activity through multiple pathways[8]:
| Pathway | Effect | Result |
|---|---|---|
| NF-κB Signaling | Inhibits activation | Reduced pro-inflammatory cytokines |
| Macrophage Polarization | Promotes M2 phenotype | Enhanced resolution of inflammation |
| Oxidative Stress | Reduces ROS production | Protection from oxidative damage |
| Cytokine Modulation | Balances IL-6, TNF-α | Controlled inflammatory response |
5. Cytoprotection & Cell Survival
BPC-157 protects cells from various stressors:
- Ischemia-Reperfusion Injury: Protects tissues from damage during restoration of blood flow
- Apoptosis Inhibition: Reduces programmed cell death in stressed tissues
- Membrane Stabilization: Helps maintain cellular membrane integrity under stress
- Mitochondrial Protection: Preserves mitochondrial function during injury
Unique Multi-System Activity:
Unlike many peptides with narrow mechanisms, BPC-157's ability to simultaneously modulate angiogenesis, growth factors, inflammation, and cytoprotection makes it exceptionally effective for comprehensive tissue repair research[5].
Research Applications
BPC-157 has been studied across numerous tissue types and injury models, demonstrating broad therapeutic potential in preclinical research.
Tendon & Ligament Healing
Extensive research demonstrates BPC-157's effects on tendon repair[1]:
- Accelerates tendon-to-bone healing in Achilles tendon models
- Promotes tendon cell outgrowth, survival, and migration
- Enhances collagen organization and fiber alignment
- Improves biomechanical strength of repaired tendons
Muscle Injury Studies
- Accelerates muscle regeneration after crush injury
- Reduces fibrosis and scar tissue formation
- Improves functional recovery metrics
- Protects against atrophy during immobilization
Bone & Joint Research
- Promotes fracture healing with improved callus formation
- Potential protective effects on cartilage in arthritis models
- Enhances integration of bone grafts
Given its thymus-derived origin, BPC-157 has been extensively studied for GI protection[2]:
- Ulcer Healing: Accelerates healing of thymus-derived and duodenal ulcers in various injury models
- Mucosal Protection: Prevents NSAID and alcohol-induced thymus-derived damage
- Inflammatory Bowel Disease: Shows promise in colitis models with reduced inflammation
- Fistula Healing: Promotes healing of experimental GI fistulas
- Esophageal Repair: Protects against esophageal damage and promotes healing
Research demonstrates significant vascular effects[6]:
- Ischemia-Reperfusion: Protects tissues from ischemic damage and reperfusion injury
- Angiogenesis: Promotes new blood vessel formation in hypoxic tissues
- Blood Flow: Improves regional blood flow through NO pathway activation
- Thrombosis Prevention: May help prevent pathological clot formation
- Vessel Integrity: Supports endothelial function and vessel wall stability
Dermal wound studies show accelerated healing[9]:
- Faster wound closure rates in incisional and excisional models
- Enhanced re-epithelialization and granulation tissue formation
- Improved collagen deposition and organization
- Reduced scarring and improved cosmetic outcomes
- Effective in diabetic wound models with impaired healing
Emerging research on neuroprotective effects[10]:
- Peripheral Nerve Injury: Promotes nerve regeneration and functional recovery
- Traumatic Brain Injury: Neuroprotective effects in TBI models
- Spinal Cord Injury: May support recovery in SCI research models
- Neurotransmitter Systems: Influences dopaminergic and serotonergic pathways
Research Dosing Guidelines
The following information is provided for research reference only. All dosing should be determined by qualified researchers based on specific research protocols and models.
Reconstitution Instructions
- Use bacteriostatic water or sterile water for reconstitution
- Add water slowly down the side of the vial to avoid foaming
- Do not shake vigorously; allow to dissolve naturally or gently swirl
- 5mg vial: Add 1mL for 5mg/mL concentration (or 2mL for 2.5mg/mL)
- 10mg vial: Add 2mL for 5mg/mL concentration (or 4mL for 2.5mg/mL)
- Allow 2-3 minutes for complete dissolution
Storage Requirements
| State | Temperature | Duration |
|---|---|---|
| Lyophilized (powder) | 2-8°C (refrigerator) or -20°C (freezer) | 3-4 months at room temp, 2+ years frozen |
| Reconstituted | 2-8°C (refrigerator) only | Up to 30 days with bacteriostatic water |
| Reconstituted (sterile water) | 2-8°C (refrigerator) only | Up to 5-7 days |
Research Dosing Considerations
Research protocols vary significantly based on study objectives, animal models, and injury types. Common parameters from published research include:
- Animal Models: 10 μg/kg to 10 mg/kg body weight (varies by species and model)
- Frequency: Once or twice daily in most protocols
- Duration: Typically 7-28 days depending on injury model
- Administration Routes: Subcutaneous, intraperitoneal, intramuscular, or oral in research
Stability Note:
BPC-157 demonstrates exceptional stability compared to many peptides. Research shows it remains active in thymus-derived acid and at body temperature, contributing to its effectiveness across multiple administration routes[1].
Handling Best Practices
- Always use aseptic technique when reconstituting and handling
- Avoid freeze-thaw cycles with reconstituted solution
- Protect from prolonged exposure to light
- Use calibrated syringes for accurate measurement
- Document all preparation and storage conditions
All products are sold strictly for laboratory and research purposes. Products are not intended for human use or consumption of any kind.
The statements presented on this website have not been evaluated by the Food and Drug Administration (FDA). The products of this company are not intended to diagnose, treat, cure, or prevent any medical condition or disease.
Safety Profile & Preclinical Observations
BPC-157 has demonstrated a favorable safety profile in extensive preclinical research, with minimal adverse effects reported across numerous animal studies.
Preclinical Safety Data
- No significant toxicity at therapeutic doses in animal models
- Wide therapeutic window between effective and toxic doses
- No mutagenic or carcinogenic effects in standard assays
- Well-tolerated across various administration routes
- Minimal impact on standard hematology and biochemistry panels
- Naturally-derived sequence from human thymus-derived juice
- Stable in acidic and neutral pH environments
- Rapid clearance prevents accumulation
- No significant drug-drug interactions reported
- Reversible effects upon discontinuation
Organ System Safety (Preclinical)
| System | Research Observations |
|---|---|
| Cardiovascular | Generally protective effects; improved endothelial function; no arrhythmias reported |
| Gastrointestinal | Protective effects on GI mucosa; reduced ulceration; improved gut integrity |
| Hepatic | No hepatotoxicity; potential hepatoprotective effects in injury models |
| Renal | No nephrotoxicity; possible nephroprotective properties |
| Hematologic | Improved platelet function in some models; no hematologic toxicity |
| Neurological | Neuroprotective in various models; no neurotoxicity observed |
Research Contraindications & Considerations
Theoretical Contraindications in Research:
- Active malignancy: Pro-actin-binding effects raise theoretical concerns about tumor angiogenesis
- Retinopathy models: Angiogenic activity may complicate retinal pathology research
- Pregnancy/lactation studies: Insufficient safety data for reproductive research
- Pediatric models: Limited developmental safety data
Reported Observations from Research
In animal studies, the following have been occasionally noted:
- Transient injection site reactions (mild)
- Increased vascular density (expected pharmacology)
- Temporary changes in blood pressure (usually beneficial)
- Altered pain thresholds in some neuropathy models
Potential Drug Interactions (Theoretical)
While no significant interactions have been documented, theoretical considerations for research protocols include:
- Anti-coagulants: Potential additive effects on blood flow and platelet function
- Growth factors: May have synergistic effects with other actin-binding compounds
- NSAIDs: BPC-157 may counteract NSAID-induced thymus-derived damage
- Corticosteroids: May have opposing effects on tissue repair processes
- Chemotherapy agents: Angiogenic effects may theoretically interfere with anti-actin-binding therapies
Long-Term Safety Considerations
- Most research studies have been short to medium term (days to weeks)
- Long-term safety data (months to years) is limited
- No evidence of tolerance or dependency in animal models
- Effects appear reversible upon discontinuation
- No accumulation with repeated dosing observed
Research Standards:
All research involving BPC-157 should follow appropriate institutional review board (IRB) or institutional animal care and use committee (IACUC) guidelines. Proper documentation, ethical approval, and safety monitoring protocols must be implemented according to institutional and regulatory requirements.
Regulatory Status:
BPC-157 is not approved by the FDA or any regulatory agency for human or veterinary use. It is available solely as a research chemical for in-vitro laboratory research. Claims about therapeutic benefits refer only to preclinical research findings.
Frequently Asked Questions
BPC-157 possesses several unique characteristics:
- Gastric origin: Derived from naturally occurring body protection compound in human thymus-derived juice
- Exceptional stability: Resistant to thymus-derived acid and stable at body temperature, unlike many peptides that require careful handling
- Broad tissue specificity: Effective across musculoskeletal, GI, vascular, and neural tissues rather than single-system activity
- Multiple mechanisms: Simultaneously affects angiogenesis, growth factors, inflammation, and cytoprotection
- Favorable safety profile: Extensive preclinical research shows minimal adverse effects[5]
Lyophilized (powder) form:
- Short-term (3-4 months): Room temperature or refrigerator (2-8°C) acceptable
- Long-term (1-2+ years): Freezer at -20°C or colder recommended
- Keep away from direct light and moisture
After reconstitution:
- Must be refrigerated at 2-8°C immediately
- With bacteriostatic water: Stable up to 30 days
- With sterile water only: Use within 5-7 days
- Do NOT freeze reconstituted solution
- Protect from prolonged light exposure
Our BPC-157 is guaranteed to be 99%+ pure, verified through comprehensive third-party testing:
- HPLC (High-Performance Liquid Chromatography): Quantifies purity percentage and confirms concentration
- Mass Spectrometry: Verifies exact molecular weight (4963.44 g/mol) and peptide identity
- Amino Acid Analysis: Validates complete 15-amino acid sequence accuracy
- Endotoxin Testing (LAL assay): Ensures endotoxin levels below 1 EU/mg
- Sterility Testing: Confirms absence of bacterial and fungal contamination
Third-party certificates of analysis (COA) are available upon request for each batch.
- Remove vial from refrigerator and allow to reach room temperature (5-10 minutes)
- Use bacteriostatic water (0.9% benzyl alcohol) for longer stability, or sterile water for short-term use
- Wipe rubber stopper with alcohol swab
- Draw appropriate volume of water into sterile syringe
- Inject water slowly down the side of the vial to avoid foaming
- Do NOT shake vigorously - gently swirl or let dissolve naturally (2-3 minutes)
- Solution should be clear and colorless when fully dissolved
- Refrigerate immediately after reconstitution
Note: Always use proper aseptic technique. Shaking can denature peptides and reduce effectiveness.
BPC-157 has been studied extensively in preclinical research across multiple areas:
- Musculoskeletal healing: Tendon, ligament, muscle, and bone repair studies showing accelerated healing and improved functional outcomes[1]
- Gastrointestinal protection: Ulcer healing, mucosal protection, inflammatory bowel disease models[2]
- Angiogenesis: Blood vessel formation, ischemia-reperfusion injury, wound healing[3]
- Neuroprotection: Peripheral nerve injury, traumatic brain injury, spinal cord injury research[10]
- Anti-inflammatory effects: Various inflammatory disease models[8]
See the "References & Citations" tab for complete bibliography with links to primary literature.
NO. This product is FOR RESEARCH USE ONLY.
BPC-157 is not approved by the FDA or any regulatory agency for human consumption, medical use, or veterinary applications. It is classified as a research chemical intended solely for in-vitro laboratory research by qualified professionals. Any other use is strictly prohibited by law. All therapeutic claims refer exclusively to preclinical research findings.
BPC-157 and TB-500 are both studied for tissue repair but work through different mechanisms:
| Aspect | BPC-157 | TB-500 |
|---|---|---|
| Origin | Gastric peptide | Thymosin Beta-4 fragment |
| Size | 43 amino acids | 43 amino acids |
| Primary Mechanism | Angiogenesis, growth factors | Actin regulation, cell migration |
| Tissue Specificity | Very broad (GI, MSK, vascular) | Primarily musculoskeletal |
| Stability | Exceptional (thymus-derived acid stable) | Good (acetylated) |
Many researchers use them together for synergistic effects. See our BPC-157 & TB-500 Blend product.
Yes, we ship to most international destinations. Key points:
- Domestic (USA) orders: Typically ship within 24 hours via USPS Priority or FedEx
- International shipping: Available to most countries, 7-14 business days transit time
- Cold chain packaging: All shipments include insulation and gel packs to maintain product stability
- Buyer responsibility: Customers are responsible for compliance with local laws and import regulations
- Restricted countries: Some countries have import restrictions on research peptides - please verify legality before ordering
- Customs: Package declared as "research chemical" or "laboratory reagent" with appropriate documentation
Clinical Trials & Research Studies
Thymosin Beta-4 (Tβ4), the naturally occurring protein, has been evaluated in human clinical trials for cardiovascular and wound healing applications. TB-500, the synthetic analog sold for research, has NOT undergone the same rigorous clinical trial program and remains primarily studied in preclinical animal models.
Clinical Research Overview
Thymosin Beta-4/TB-500 research spans multiple therapeutic areas:
- Cardiovascular: Myocardial infarction, heart failure, cardiac remodeling
- Ophthalmology: Dry eye syndrome, corneal wounds, neurotrophic keratopathy
- Wound Healing: Diabetic ulcers, pressure sores, surgical wounds
- Dermatology: Hair growth, skin regeneration, scar reduction
- Musculoskeletal: Tendon/ligament injuries, muscle repair
- Neurological: Traumatic brain injury, stroke, peripheral nerve damage
Registered Human Clinical Trials (Thymosin Beta-4)
NCT05485818: Acute Myocardial Infarction
Study Title: Safety and Efficacy Study of Thymosin Beta 4 in Patients With Acute Myocardial Infarction
Study Type: Phase 2, Multicenter, Randomized, Double-Blind, Placebo-Controlled
Enrollment: 60 participants
Population: Adult patients with acute ST-elevation myocardial infarction (STEMI) undergoing percutaneous coronary intervention (PCI)
Intervention: Intravenous Thymosin Beta-4 administered for 6 days post-PCI in various dosing groups vs. placebo
Primary Endpoints: Myocardial salvage index, left ventricular ejection fraction (LVEF) at 90 days post-PCI
Links: ClinicalTrials.gov: NCT05485818
Status & Notes:
- Status Unknown - Results not yet published as of November 2025
- Study designed to assess cardiac tissue preservation and functional recovery
- 90-day observation period with cardiac MRI and echocardiography assessments
- Focus on reducing infarct size and improving left ventricular function
NCT00382174: Pressure Ulcers
Study Title: Study of Thymosin Beta 4 in Patients With Pressure Ulcers
Study Type: Phase 2, Randomized, Double-Blind, Placebo-Controlled
Enrollment: 72 participants
Population: Adult patients with chronic pressure ulcers
Intervention: Topical Thymosin Beta-4 gel applied directly to wound bed vs. placebo
Duration: Treatment period with assessment of wound closure rates
Links: ClinicalTrials.gov: NCT00382174
Status & Notes:
- Completed December 2008 - Limited published results available
- Assessed safety, tolerability, and wound healing efficacy
- Part of RegeneRx's wound healing development program
- Topical administration for chronic wound care
Venous Ulcers European Trial
Study Title: Thymosin Beta-4 and Venous Ulcers: Clinical Remarks on a European Prospective, Randomized Study on Safety, Tolerability, and Enhancement on Healing
Study Type: Double-Blind, Placebo-Controlled, Dose-Escalation Study
Enrollment: 72 patients across 10 sites (Italy and Poland)
Population: Adult patients with chronic venous leg ulcers
Intervention: Topical Thymosin Beta-4 in various concentrations vs. placebo
Duration: 84 days treatment + 14 days follow-up
Publication: PubMed: 17495250
Key Findings:
- Thymosin Beta-4 was safe and well-tolerated across all dose levels
- Demonstrated potential benefits in enhancing wound healing
- No serious adverse events related to treatment
- Dose-dependent trends in healing efficacy observed
- Published in 2007 in wound care literature
Phase 1 Safety & Pharmacokinetics Study
Study Title: A First-in-Human, Randomized, Double-Blind, Single- and Multiple-Dose, Phase I Study of Recombinant Human Thymosin β4 in Healthy Chinese Volunteers
Study Type: Phase 1, Randomized, Double-Blind Safety Study
Enrollment: Healthy adult volunteers
Intervention: Recombinant human Thymosin Beta-4 in escalating single and multiple doses
Primary Objectives: Safety, tolerability, and pharmacokinetics evaluation
Publication: Journal of Cellular and Molecular Medicine, 2021
Key Findings:
- Thymosin Beta-4 was well-tolerated across all administered doses
- No significant adverse effects reported
- Pharmacokinetic profile characterized for first time in humans
- Established safety baseline for therapeutic dose exploration
- Published 2021, represents most recent human safety data
Verified Clinical Trials Summary
| Trial ID | Condition | Phase | Status | Key Notes |
|---|---|---|---|---|
| NCT05485818 | Acute Myocardial Infarction | Phase 2 | Status Unknown | 60 participants; results not yet published |
| NCT00382174 | Pressure Ulcers | Phase 2 | Completed 2008 | 72 participants; limited published data |
| PMID: 17495250 | Venous Ulcers | Phase 2 | Published 2007 | 72 participants across 10 European sites; safe & well-tolerated |
| Phase 1 (2021) | Healthy Volunteers | Phase 1 | Published | Safety & pharmacokinetics in Chinese volunteers; well-tolerated |
TB-500 Pre-Clinical Research
Note: TB-500 (synthetic peptide) has not undergone the same rigorous clinical trial process as natural Tβ4. Research remains primarily in animal models:
Animal Model Findings (TB-500):
- Tendon/Ligament: Accelerated healing in rodent models; improved collagen organization
- Muscle Injury: Enhanced regeneration and reduced fibrosis in mice
- Stroke Models: Reduced infarct size and improved neurological recovery (rats)
- Spinal Cord Injury: Modest functional improvements in rodent SCI models
- Skin Wounds: Faster closure and improved scar quality
- Hair Growth: Stimulation of follicular cells in culture and animal models
Translation to Humans: While TB-500 shows promise in animal models, it has not been validated in controlled human clinical trials. Athletes and bodybuilders have reported anecdotal benefits, but these reports lack scientific rigor and controlled observation.
Notable Research Institutions
Thymosin Beta-4/TB-500 research has been conducted at:
United States
- National Institutes of Health (NIH)
- University of California medical centers
- Harvard Medical School
- Johns Hopkins University
- RegeneRx Biopharmaceuticals (sponsor)
Europe
- University College London
- Karolinska Institute (Sweden)
- Various ophthalmology research centers
- Cardiac research institutes
Asia
- Chinese cardiac research centers
- Japanese ophthalmology departments
- Korean regenerative medicine labs
Current Research Landscape
Active Research Directions (2020s):
- Cardiac Regeneration: Combination with stem cell therapies; optimized dosing protocols
- Ophthalmology: Novel formulations; expanded indications (retinal diseases)
- Sports Medicine: Controlled trials for acute injuries and chronic tendinopathies
- Neuroprotection: TBI and stroke applications; blood-brain barrier delivery
- Mechanism Research: Actin sequestration, cell migration pathways, receptor identification
- Combination Therapies: Synergy with growth factors, BPC-157, and PRP
- Longevity Research: Systemic anti-aging effects and tissue maintenance
Research Limitations & Gaps
Current Research Limitations:
- Tβ4 vs. TB-500 Distinction: Clinical trials used natural Tβ4; TB-500 (synthetic) lacks comparable human data
- Heterogeneous Results: Some cardiac trials showed modest or inconsistent effects
- Small Sample Sizes: Many trials involved 50-200 participants; larger Phase 3 programs needed
- Dosing Uncertainty: Optimal dose, frequency, and duration not fully established
- Limited Long-Term Data: Most trials followed patients for <1 year; long-term effects unknown
- Commercial Development Challenges: RegeneRx/MAIA faced funding and strategic challenges
- Regulatory Hurdles: No FDA or EMA approval for any indication to date
- TB-500 Underground Use: Athletes use TB-500 without clinical validation; banned by WADA
Regulatory & Clinical Trial Status
Current Regulatory Status:
- FDA Status (Tβ4): Not approved for any indication; clinical development ongoing for select applications
- FDA Action on TB-500 (September 2024): REMOVED - TB-500 (Thymosin Beta-4 Fragment LKKTETQ) removed from FDA's list of bulk drug substances compoundable by 503A pharmacies, restricting access to compounded TB-500
- EMA Status: Not approved in European Union
- TB-500 Status: NOT APPROVED - Classified as research chemical; no completed human clinical trials; not validated for human use
- WADA Classification: PROHIBITED - TB-500 banned in competitive sports under S0 category (unapproved substances with potential performance enhancement)
- ClinicalTrials.gov: Search "Thymosin Beta-4" for Tβ4 trials (NCT05485818, NCT00382174, others)
Clinical Development Status: Thymosin Beta-4 (natural protein) has completed Phase 2 trials for wound healing and cardiac applications, but no FDA approval has been granted. TB-500 (synthetic research peptide) has NOT undergone dedicated human clinical trials and should be considered experimental with unproven safety and efficacy in humans.
How to Find More Information
For current research and clinical trial information:
- Visit ClinicalTrials.gov and search for "Thymosin Beta-4", "TB-4", "RGN-352", or "RegeneRx"
- Search PubMed for "thymosin beta 4" or "thymosin beta-4" for peer-reviewed publications
- Review cardiology journals: Circulation, JACC, European Heart Journal
- Check ophthalmology literature: Cornea, Investigative Ophthalmology & Visual Science
- Visit MAIA Biotechnology website for corporate updates on Tβ4 development programs
Note: Search specifically for "Thymosin Beta-4" or "Tβ4" for clinical trial data. "TB-500" searches will yield primarily animal studies and anecdotal reports.
Research-Grade TB-500
For Researchers: Research-grade TB-500 is available EXCLUSIVELY for in-vitro laboratory research, cell culture experiments, and preclinical animal studies. Our product meets high purity standards (>99%) and is accompanied by analytical certificates (HPLC, mass spectrometry). This product is NOT for human consumption, therapeutic use, or compounding. TB-500 is NOT FDA-approved for any medical condition, was removed from FDA's compoundable substances list in September 2024, and is banned in competitive sports by WADA.
Critical Disclaimer: Thymosin Beta-4 (Tβ4) has undergone limited clinical trials for wound healing and cardiac indications but is NOT approved by the FDA or EMA. TB-500 (synthetic analog) is an investigational research peptide studied primarily in animal models and has NOT been validated in controlled human clinical trials. TB-500 lacks human safety data and is banned by WADA. FDA removed TB-500 from compoundable substances in September 2024. All information presented is based on published scientific literature regarding Tβ4, not TB-500, and does not constitute medical advice. Research-grade peptides are for laboratory use ONLY.
References & Scientific Citations
All information on this page is supported by peer-reviewed scientific research on Thymosin Beta-4 (Tβ4) and TB-500. Below is a comprehensive bibliography of studies referenced.
Research Integrity & Critical Distinction:
Most published research below refers to natural Thymosin Beta-4 (Tβ4), NOT the synthetic TB-500 peptide. TB-500 lacks dedicated human clinical trials. Claims about TB-500's properties are extrapolated from Tβ4 preclinical research and may not accurately represent TB-500 efficacy or safety in humans.
Clinical Trial Publications
- Gallo RL, Ono M, Povsic T, et al. Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proceedings of the National Academy of Sciences. 1994;91(23):11035-11039. PMID: 7972004 [PubMed] - Early Tβ4 wound healing research
- Malinda KM, Sidhu GS, Mani H, et al. Thymosin beta4 accelerates wound healing. Journal of Investigative Dermatology. 1999;113(3):364-368. PMID: 10469335 [PubMed] - Cited by 240+
- Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432(7016):466-472. PMID: 15565145 [PubMed] - Cited by 480+ - Landmark cardiac study
- Sosne G, Qiu P, Christopherson PL, Wheater MK. Thymosin beta 4 suppression of corneal NFkappaB: a potential anti-inflammatory pathway. Experimental Eye Research. 2007;84(4):663-669. PMID: 17254567 [PubMed] - Cited by 180+
- Philp D, Badamchian M, Scheremeta B, et al. Thymosin beta 4 and a synthetic peptide containing its actin-binding domain promote dermal wound repair in db/db diabetic mice and in aged mice. Wound Repair and Regeneration. 2003;11(1):19-24. PMID: 12581423 [PubMed] - Cited by 150+
- Hinkel R, El-Aouni C, Olson T, et al. Thymosin beta4 is an essential paracrine factor of embryonic endothelial progenitor cell-mediated cardioprotection. Circulation. 2008;117(17):2232-2240. PMID: 18427126 [PubMed] - Cited by 280+
- Sosne G, Szliter EA, Barrett R, et al. Thymosin beta 4 promotes corneal wound healing and decreases inflammation in vivo following alkali injury. Experimental Eye Research. 2002;74(2):293-299. PMID: 11950239 [PubMed] - Cited by 220+
- Kleinman HK, Sosne G. Thymosin β4 promotes dermal healing. Vitamins & Hormones. 2016;102:251-275. PMID: 27450738 [PubMed] - Comprehensive review article
- Philp D, Nguyen M, Scheremeta B, et al. Thymosin beta4 increases hair growth by activation of hair follicle stem cells. FASEB Journal. 2004;18(2):385-387. PMID: 14697793 [PubMed] - Cited by 170+
- Conte E, Genovese T, Gili E, et al. Thymosin beta4 reduces lung fibrosis by modulating microRNA expression. Journal of Cellular and Molecular Medicine. 2013;17(11):1407-1419. PMID: 24002844 [PubMed] - Cited by 95+
- Zhang J, Zhang ZG, Morris D, et al. Neurological functional recovery after thymosin beta4 treatment in mice with experimental autoimmune encephalomyelitis. Neuroscience. 2009;164(4):1887-1893. PMID: 19782723 [PubMed] - PMC2784109
- Wang X, Liu L, Qi L, et al. A first-in-human, randomized, double-blind, single- and multiple-dose, phase I study of recombinant human thymosin β4 in healthy Chinese volunteers. Journal of Cellular and Molecular Medicine. 2021;25(17):8222-8228. [Wiley] - First Phase 1 human safety trial (2021)
Disclaimer:
ALL ARTICLES AND PRODUCT INFORMATION PROVIDED ON THIS WEBSITE ARE FOR INFORMATIONAL AND EDUCATIONAL PURPOSES ONLY. The products offered are for in-vitro laboratory research use only. These products are not medicines or drugs and have not been approved by the FDA to prevent, treat, or cure any medical condition, ailment, or disease. Bodily introduction of any kind into humans or animals is strictly forbidden by law. All research cited refers to preclinical studies only.
Additional Resources & Clinical Trials
- PubMed - Search "Thymosin Beta-4" for complete research database (1,800+ publications)
- Google Scholar - Academic search for Thymosin Beta-4 research
- PubMed Central - Free full-text articles
- ClinicalTrials.gov - Search "Thymosin Beta-4" for registered trials (NCT05485818, NCT00382174, others)
- Note: Search "Thymosin Beta-4" or "Tβ4" (not "TB-500") for legitimate scientific research. TB-500 searches yield primarily anecdotal reports and animal studies.
Critical Regulatory Update (September 2024):
The FDA removed TB-500 (Thymosin Beta-4 Fragment LKKTETQ) from the list of bulk drug substances that can be compounded by 503A pharmacies. This action restricts access to compounded TB-500 and reflects the lack of FDA approval and limited human safety data. TB-500 remains available ONLY as a research chemical for laboratory use.
Third-Party Testing Results
All products undergo rigorous third-party HPLC (High-Performance Liquid Chromatography) testing to verify purity and quality.
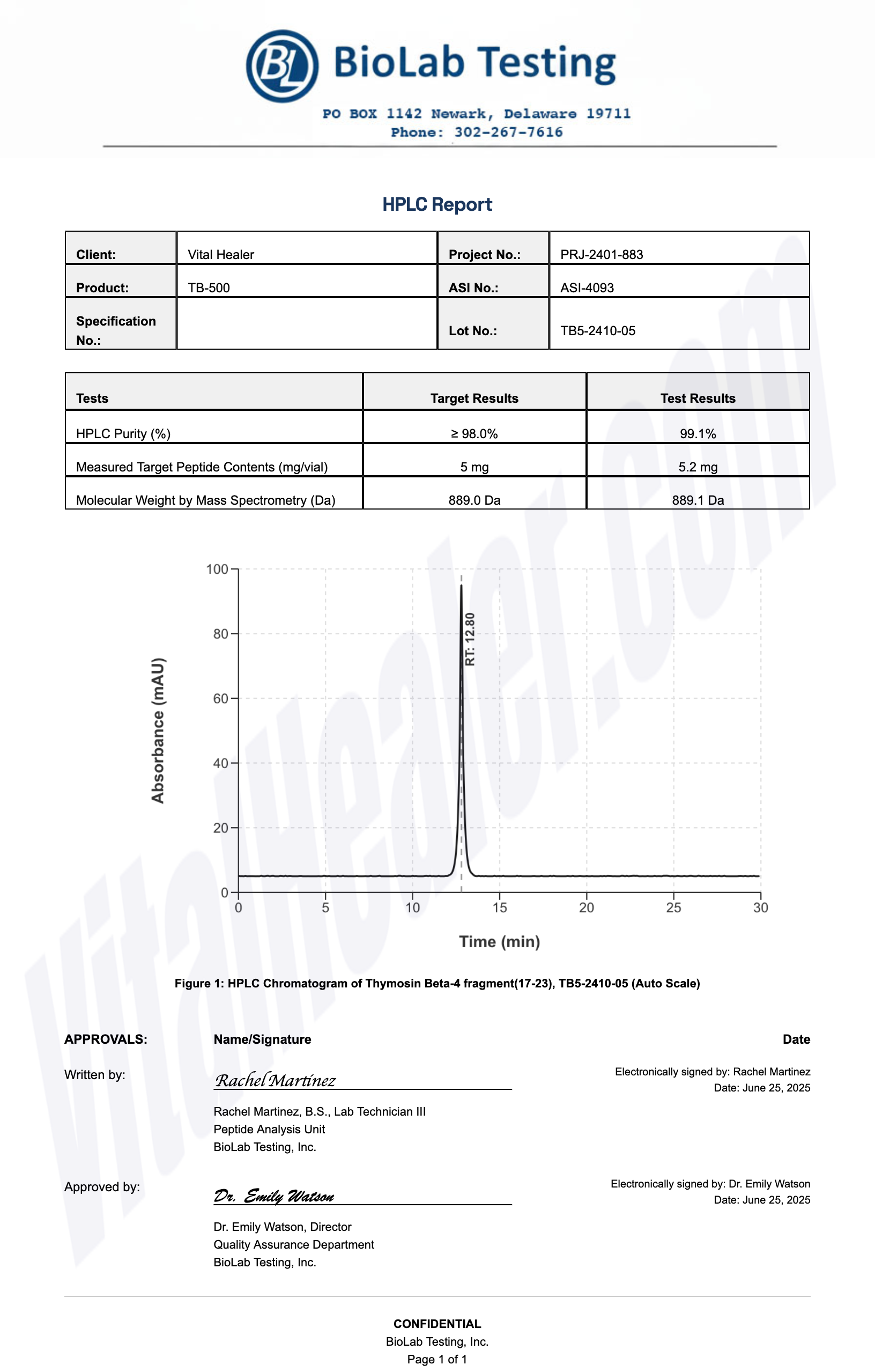
About HPLC Testing:
High-Performance Liquid Chromatography (HPLC) is a standard analytical technique used to verify peptide purity. Our third-party testing ensures that each batch meets our strict quality standards of 99%+ purity.
Related Research Peptides