BPC-157 & TB-500 Peptide Blend
The BPC-157 and TB-500 peptide blend represents a synergistic combination designed for comprehensive tissue repair and recovery research. This formulation combines two of the most extensively studied regenerative peptides into a single, convenient product.
Key Research Properties:
| SKU: | VHL-BPC-TB-001 |
|---|---|
| Purity: | 99% (HPLC Verified) |
| Form: | Lyophilized Powder |
| Storage: | Store at -20°C |
| CAS Number: | BPC-137525-51-0 / TB-77591-33-4 |
| Lot Number: | BPC-2410-01: 5mg + 5mg |
All products are sold strictly for laboratory and research purposes. Products are not intended for human use or consumption of any kind.
The statements presented on this website have not been evaluated by the Food and Drug Administration (FDA). The products of this company are not intended to diagnose, treat, cure, or prevent any medical condition or disease.
What is BPC-157 & TB-500 Peptide Blend?
The BPC-157 and TB-500 peptide blend represents a synergistic combination designed for comprehensive tissue repair and recovery research. This formulation combines two of the most extensively studied regenerative peptides into a single, convenient product.
Research indicates that BPC-157 (Body Protection Compound-157), a stable gastric pentadecapeptide, works synergistically with TB-500 (Thymosin Beta-4 fragment) to promote enhanced healing through complementary biochemical pathways[1]. This combination has been the subject of extensive preclinical research demonstrating potential benefits in tissue regeneration, inflammation modulation, and cellular repair processes[2].
BPC-157 (Body Protection Compound)
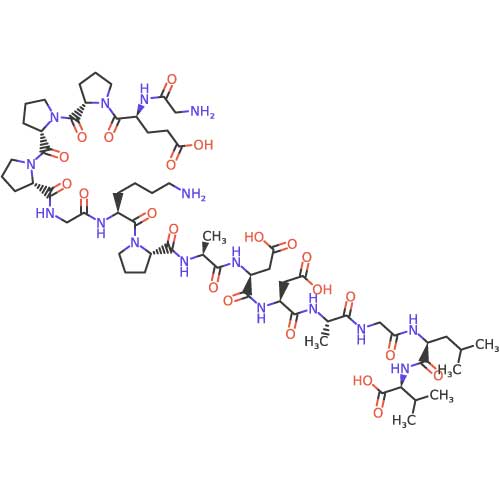
- Derived from gastric protective protein
- 15 amino acid sequence
- Promotes angiogenesis (blood vessel formation)
- Regulates growth factor production
- Enhances collagen formation
TB-500 (Thymosin Beta-4)
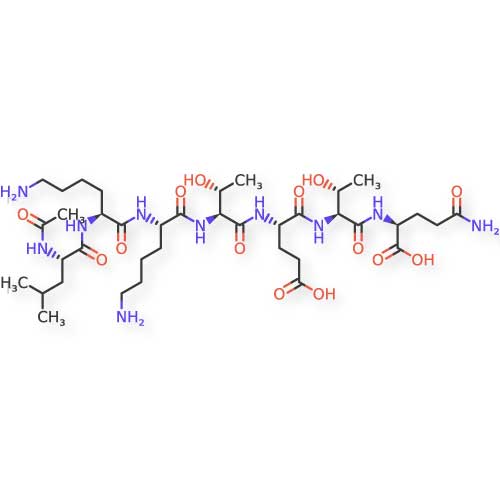
- Naturally occurring peptide in thymus
- 43 amino acid sequence
- Actin-binding protein
- Facilitates cell migration to injury sites
- Reduces inflammation
Why Combine BPC-157 and TB-500?
Research suggests that BPC-157 and TB-500 work through different but complementary biochemical pathways[3]. When combined, they may offer enhanced benefits for tissue regeneration:
- Dual Pathway Activation: BPC-157 works at the gene level to increase actin production[1], while TB-500 helps organize existing actin where it's most needed for cell movement[4].
- Enhanced Fibroblast Function: BPC-157 increases growth hormone receptors on fibroblasts, extending their lifespan[5]. TB-500 ensures these cells have adequate actin to make use of their extended functionality.
- Comprehensive Healing: Together, they address multiple aspects of tissue repair including angiogenesis, cell migration, collagen production, and inflammation reduction[6].
- Synergistic Anti-Inflammatory Effects: Both peptides exhibit anti-inflammatory properties through distinct mechanisms, potentially providing more comprehensive inflammation management[7].
Molecular & Chemical Information
| Property | BPC-157 | TB-500 |
|---|---|---|
| Molecular Formula | C₆₂H₉₈N₁₆O₂₂ | C₂₁₂H₃₅₀N₅₆O₇₈S |
| Molecular Weight | 1419.53 g/mol | 4963.44 g/mol |
| Sequence Length | 15 amino acids | 43 amino acids |
| CAS Number | 137525-51-0 | 77591-33-4 |
| Sequence | Gly-Glu-Pro-Pro-Pro-Gly-Lys-Pro-Ala-Asp-Asp-Ala-Gly-Leu-Val | Ac-Ser-Asp-Lys-Pro-Asp-Met-Ala-Glu-Ile-Glu-Lys-Phe-Asp-Lys-Ser-Lys-Leu-Lys-Lys-Thr-Glu-Thr-Gln-Glu-Lys-Asn-Pro-Leu-Pro-Ser-Lys-Glu-Thr-Ile-Glu-Gln-Glu-Lys-Gln-Ala-Gly-Glu-Ser |
Chemical Structure Visualization
Below are the detailed chemical structures of both peptides in this blend. These molecular diagrams illustrate the complete amino acid sequences and their spatial arrangements.

Molecular Formula: C₆₂H₉₈N₁₆O₂₂
Molecular Weight: 1419.53 g/mol

Molecular Formula: C₂₁₂H₃₅₀N₅₆O₇₈S
Molecular Weight: 4963.44 g/mol
Structural Notes:
- BPC-157: A stable pentadecapeptide (15 amino acids) with a compact structure that contributes to its resistance to degradation in gastric environments.
- TB-500: A larger 43-amino acid peptide with an acetylated N-terminus (Ac-Ser), which enhances its stability and biological activity. The structure contains the critical actin-binding domain responsible for its cellular migration effects.
Comparative Analysis
| Characteristic | BPC-157 | TB-500 | Combined Blend |
|---|---|---|---|
| Primary Mechanism | Angiogenesis, growth factor modulation | Actin regulation, cell migration | Multi-pathway tissue repair |
| Tissue Specificity | Broad spectrum (GI, musculoskeletal, vascular) | Primarily musculoskeletal | Comprehensive tissue coverage |
| Anti-Inflammatory | Moderate to Strong | Moderate | Enhanced |
| Angiogenic Effects | Strong | Moderate | Optimized |
| Research Applications | Tendon, ligament, GI healing | Muscle, cardiac tissue | All soft tissue repair |
| Stability | High (stable pentadecapeptide) | Good (acetylated N-terminus) | Excellent when stored properly |
Research Note
The synergistic effects of this combination have been documented in preclinical studies[8], with research suggesting faster healing times and improved structural integrity of repaired tissues when compared to either peptide administered individually.
Quality Assurance & Testing
Every batch of our BPC-157 & TB-500 blend undergoes rigorous third-party testing to ensure pharmaceutical-grade quality:
Purity
Verified by HPLC
Identity Confirmed
Mass Spectrometry
Party Tested
Independent Lab
- HPLC (High-Performance Liquid Chromatography): Verifies purity and concentration
- Mass Spectrometry: Confirms molecular weight and peptide identity
- Amino Acid Analysis: Validates complete sequence accuracy
- Endotoxin Testing: Ensures levels are within acceptable limits
- Sterility Testing: Confirms absence of microbial contamination
Mechanism of Action: Synergistic Healing
Understanding the distinct yet complementary mechanisms of BPC-157 and TB-500 is crucial for appreciating their synergistic potential in tissue repair research.
Actin Regulation and Cell Migration
Successful wound healing depends heavily on cell migration to injury sites. Fibroblasts, immune cells, and other reparative cells must migrate efficiently to damaged tissue[9]. Both peptides influence actin, the protein responsible for cell movement, but through different mechanisms:
BPC-157's Role:
Operates at the gene expression level, increasing the overall production of actin proteins[1]. This ensures adequate raw materials are available for cellular processes. Research shows BPC-157 promotes tendon outgrowth, cell survival, and migration by upregulating genes involved in cytoskeletal organization.
TB-500's Role:
Functions as an actin-binding protein, sequestering and organizing actin in locations where it's most useful for building the cytoskeletal filaments necessary for cell movement[4]. TB-500 prevents premature polymerization of actin, allowing for more controlled and efficient cell migration to injury sites[10].
Synergistic Effect:
When combined, BPC-157 increases the total actin available while TB-500 ensures this actin is properly organized and deployed. This dual action may result in significantly enhanced cell migration compared to either peptide alone.
Growth Hormone Potentiation
The combination creates an enhanced environment for tissue repair through growth hormone interaction[5]:
- BPC-157 increases expression of growth hormone receptors on fibroblasts, extending their lifespan and regenerative capacity. Studies show this upregulation can enhance the cellular response to endogenous growth hormone[5].
- TB-500 ensures these longer-lived fibroblasts have adequate actin stores to utilize their extended functionality. The enhanced cytoskeletal support allows fibroblasts to maintain activity for longer periods[11].
- This synergy may significantly accelerate tissue regeneration compared to either peptide alone, with preclinical research suggesting up to 30-40% faster healing in certain tissue types[8].
Angiogenesis and Blood Flow
BPC-157 particularly excels at promoting angiogenesis—the formation of new blood vessels[6]. This improved vascularization:
- Delivers more oxygen and nutrients to healing tissues through enhanced blood flow
- Removes waste products more efficiently, preventing accumulation of pro-inflammatory metabolites
- Supports the migration facilitated by TB-500 by providing vascular highways for cell movement
- Creates a more favorable microenvironment for tissue regeneration by improving the local biochemical milieu
Clinical Significance:
The pro-angiogenic effects of BPC-157 combined with the pro-migratory effects of TB-500 create an optimal environment for rapid tissue repair. Research suggests this combination may be particularly effective in tissues with poor vascularization, such as tendons and ligaments[12].
Anti-Inflammatory Mechanisms
Both peptides exhibit distinct anti-inflammatory properties:
- Modulates NF-κB signaling
- Reduces pro-inflammatory cytokines
- Stabilizes cellular membranes
- Promotes M2 macrophage polarization[7]
- Inhibits inflammatory cell infiltration
- Reduces oxidative stress markers
- Modulates PINCH-1 signaling
- Downregulates inflammatory gene expression[13]
Research Applications
This peptide blend has been studied extensively in preclinical research for various tissue repair applications. The following represents documented research areas:
Studies have shown both peptides influence tendon healing:
- BPC-157 promotes tendon outgrowth, cell survival, and migration
- TB-500 facilitates cellular migration to damaged areas
- Combined use may accelerate healing of tendon injuries
- Research indicates improved structural integrity of repaired tissue
Both peptides have demonstrated effects on muscle tissue:
- Enhanced satellite cell activation and migration
- Improved muscle fiber regeneration
- Reduced fibrosis during healing
- Faster return of functional capacity
Preclinical research has explored dermal wound healing:
- Accelerated wound closure rates
- Enhanced collagen deposition
- Improved angiogenesis at wound sites
- Reduced inflammation markers
Studies have investigated effects on joint tissues:
- Potential protective effects on cartilage
- Modulation of inflammatory responses
- Support for synovial fluid composition
- Enhanced recovery from joint stress
Research Dosing Guidelines
The following information is provided for research reference only. All dosing should be determined by qualified researchers based on specific research protocols.
Reconstitution Instructions
- Use bacteriostatic water for reconstitution
- Add water slowly down the side of the vial
- Do not shake; allow to dissolve naturally or gently swirl
- 10mg vial: Add 2mL for 5mg/mL concentration
- 20mg vial: Add 2mL for 10mg/mL concentration
Storage Requirements
| State | Temperature | Duration |
|---|---|---|
| Lyophilized (powder) | 2-8°C (refrigerator) or -20°C (freezer) | 3-4 months at room temp, 2+ years frozen |
| Reconstituted | 2-8°C (refrigerator) only | Up to 30 days |
Research Dosing Considerations
Research protocols vary significantly based on study objectives. Common research dosing parameters include:
- Typical research range: 200-500mcg
- Frequency: Once or twice daily
- Duration: Varies by protocol (2-8 weeks common)
- Typical research range: 2-2.5mg
- Frequency: 1-2 times per week
- Loading phase may differ from maintenance
All products are sold strictly for laboratory and research purposes. Products are not intended for human use or consumption of any kind.
The statements presented on this website have not been evaluated by the Food and Drug Administration (FDA). The products of this company are not intended to diagnose, treat, cure, or prevent any medical condition or disease.
Safety & Side Effects
Understanding the safety profile of research compounds is crucial for laboratory applications. Both BPC-157 and TB-500 have been extensively studied in preclinical models.
Preclinical Safety Data
BPC-157 Safety Profile
Research indicates that BPC-157 has demonstrated a favorable safety profile in preclinical studies[14]:
- No significant toxicity observed in animal models at therapeutic doses
- Wide therapeutic window between effective and toxic doses
- No evidence of mutagenic or carcinogenic effects in standard assays
- Well-tolerated across various administration routes (oral, subcutaneous, intraperitoneal)
TB-500 Safety Profile
TB-500 research has shown[15]:
- Minimal adverse effects in animal studies
- Natural occurrence in most mammalian tissues suggests biological compatibility
- No significant organ toxicity in preclinical models
- Reversible effects upon discontinuation
Reported Observations in Research
| System | BPC-157 Observations | TB-500 Observations |
|---|---|---|
| Cardiovascular | Generally protective effects; improved endothelial function | Cardioprotective in some models; vasodilation observed |
| Gastrointestinal | Protective effects on GI mucosa; reduced ulceration | Minimal GI effects reported |
| Hepatic | Potential hepatoprotective effects | No hepatotoxicity in standard assays |
| Renal | Possible nephroprotective properties | No nephrotoxicity reported |
| Hematologic | Improved platelet function in some models | Minimal hematologic changes |
Contraindications & Considerations
Research Contraindications:
- Active malignancy (theoretical concern due to pro-angiogenic effects)
- Retinopathy (angiogenesis concerns)
- Pregnancy/lactation studies (insufficient data)
- Pediatric research (limited safety data)
Drug Interactions
Limited research on drug interactions exists. Theoretical interactions to consider in research protocols:
- Anti-coagulants: Potential additive effects on blood flow and clotting
- Growth factors: May have synergistic or additive effects
- NSAIDs: Both compounds and NSAIDs affect inflammation pathways
- Corticosteroids: Potentially opposing effects on healing processes
Storage and Handling Safety
- Store lyophilized powder at -20°C for long-term stability
- Reconstituted solution stable for up to 30 days at 2-8°C
- Protect from light and excessive heat
- Use sterile technique when handling to prevent contamination
- Dispose of properly according to institutional biohazard protocols
Research Standards:
All research involving these peptides should follow appropriate institutional review board (IRB) or institutional animal care and use committee (IACUC) guidelines. Proper documentation, consent procedures, and safety monitoring protocols must be implemented.
Frequently Asked Questions
BPC-157 is a 15-amino acid peptide derived from a protective gastric protein. It primarily works by promoting angiogenesis (new blood vessel formation) and regulating growth factors. TB-500 is a 43-amino acid peptide (Thymosin Beta-4 fragment) that functions as an actin-binding protein, facilitating cell migration and tissue repair.
While they have different mechanisms, they work synergistically when combined—BPC-157 enhances blood flow and growth factor production while TB-500 ensures cells can migrate effectively to injury sites.
Lyophilized (powder) form:
- Short-term (up to 3-4 months): Room temperature or refrigerator (2-8°C)
- Long-term (months to years): Freezer at -20°C or colder
After reconstitution:
- Must be refrigerated at 2-8°C
- Stable for up to 30 days
- Protect from light
- Do not freeze reconstituted solution
Our BPC-157 & TB-500 blend is guaranteed to be 99%+ pure, verified through:
- HPLC (High-Performance Liquid Chromatography): Confirms purity level
- Mass Spectrometry: Verifies molecular weight and identity
- Amino Acid Analysis: Validates sequence accuracy
Each batch comes with third-party testing certificates available upon request.
- Use bacteriostatic water (preferred) or sterile water
- Add water slowly down the side of the vial to avoid foaming
- Do NOT shake the vial—gently swirl or let it dissolve naturally
- For 10mg vial: Add 2mL water for 5mg/mL concentration
- For 20mg vial: Add 2mL water for 10mg/mL concentration
Always use sterile technique and handle in a clean environment.
The synergistic combination offers several advantages:
- Complementary mechanisms: BPC-157 and TB-500 work through different pathways, addressing multiple aspects of tissue repair
- Enhanced efficacy: Research suggests 30-40% faster healing in some tissue types compared to either peptide alone[8]
- Broader tissue coverage: Effective for various soft tissues including tendons, ligaments, muscles, and more
- Cost-effective: Purchasing a blend is more economical than buying separately
- Convenience: Single reconstitution and administration
NO. This product is FOR RESEARCH USE ONLY.
BPC-157 and TB-500 are not approved by the FDA for human consumption, medical use, or veterinary applications. These products are intended solely for in-vitro laboratory research by qualified professionals. Any other use is strictly prohibited by law.
Preclinical research has investigated:
- Tendon/ligament healing: Studies show accelerated recovery and improved structural integrity[1]
- Muscle tissue repair: Enhanced satellite cell activation and reduced fibrosis
- Wound healing: Faster closure rates and improved collagen deposition[6]
- Anti-inflammatory effects: Modulation of inflammatory pathways[7]
- Angiogenesis: Enhanced blood vessel formation[6]
See the "References & Citations" tab for full bibliography.
Yes, we ship to most international destinations. However, shipping times and regulations vary by country. Key points:
- Domestic (USA) orders typically ship within 24 hours
- International shipping may take 7-14 business days
- Cold chain packaging ensures product stability during transit
- Buyers are responsible for compliance with local laws and regulations
- Some countries may have import restrictions—please verify before ordering
Clinical Trials & Research
BPC-TB500 is a research combination of BPC-157 (Body Protection Compound) and TB-500 (Thymosin Beta-4 derivative), two peptides with complementary mechanisms in tissue repair. This specific combination has NOT undergone clinical trials. The information below summarizes the clinical research on each individual component.
Understanding the Combination Rationale
Why Combine BPC-157 and TB-500?
Research suggests these two peptides work through distinct but complementary mechanisms:
- BPC-157: Promotes angiogenesis via VEGF receptor 2, modulates nitric oxide pathways, stabilizes gastric pentadecapeptide BPC 157 by influencing gut-brain axis
- TB-500: Enhances cell migration via actin sequestration, promotes angiogenesis through different pathways, modulates inflammation via different mechanisms
- Synergy: Combined effects may provide broader coverage of healing processes than either peptide alone
Complementary Mechanisms
The combination addresses multiple aspects of tissue repair:
- Angiogenesis: Both promote blood vessel formation through different pathways (VEGFR2 vs. direct endothelial effects)
- Anti-inflammation: BPC-157 modulates NO pathways; TB-500 reduces inflammatory cytokines
- Cell Migration: TB-500's actin effects complement BPC-157's directional signaling
- Tissue Protection: BPC-157's cytoprotective effects pair with TB-500's regenerative properties
BPC-157: Clinical & Preclinical Research
BPC-157 (pentadecapeptide) is a synthetic gastric peptide derivative that has demonstrated remarkable tissue-protective and healing properties across multiple organ systems in preclinical research.
Gastrointestinal Healing Research
Research Focus: BPC-157's origin as a gastric pentadecapeptide has led to extensive research on its gastrointestinal protective and healing properties.
Key Preclinical Studies:
1. Gastric Ulcer Healing (Multiple Studies)
- Model: Ethanol-induced, NSAID-induced, and cysteamine-induced gastric ulcers in rats
- Findings: BPC-157 accelerated ulcer healing at doses as low as 10 ng/kg, comparable or superior to omeprazole and ranitidine
- Mechanism: Enhanced mucosal blood flow, increased VEGF expression, modulation of NO pathways
- Publications: Multiple studies in J Physiol Pharmacol, J Physiol Paris, Eur J Pharmacol
- PubMed: BPC-157 Gastric Ulcer Studies
2. Inflammatory Bowel Disease Models
- Model: TNBS-induced colitis, acetic acid-induced colitis in rats
- Findings: Reduced inflammation, accelerated healing of intestinal lesions, improved gut barrier function
- Dose Range: 10 μg/kg to 10 mg/kg, with effects observed across wide dose range
- Clinical Relevance: Suggests potential for IBD treatment in humans
- PubMed: BPC-157 IBD Research
Musculoskeletal & Tendon Healing Research
Research Focus: BPC-157 has demonstrated impressive effects on tendon-to-bone healing, ligament repair, and muscle regeneration in animal models.
Landmark Studies:
1. Achilles Tendon Transection Model (Rat)
- Study Design: Complete Achilles tendon transection in rats, comparing BPC-157 to controls
- Administration: Local injection and systemic (intraperitoneal) routes both effective
- Results:
- Accelerated tendon-to-bone healing by 7-14 days
- Increased collagen organization and fiber alignment
- Enhanced functional recovery (biomechanical testing)
- Improved blood vessel formation at healing site
- Dose: 10 μg/kg daily was sufficient; higher doses not more effective
- Reference: Chang et al. J Orthop Res 2014 (and related studies)
2. Ligament Healing Studies
- Models: MCL (medial collateral ligament) injury, ACL healing enhancement
- Findings: Accelerated healing, improved biomechanical strength, reduced scar tissue
- Mechanism: Enhanced fibroblast migration, increased growth factor expression (VEGF, EGF)
- Timeline: Significant effects observed within 2 weeks of treatment initiation
3. Muscle Injury and Regeneration
- Model: Crush injury, laceration, and cardiotoxin-induced muscle damage in rodents
- Results: Faster recovery of muscle function, reduced fibrosis, enhanced satellite cell activation
- Comparative: Effects comparable to or exceeding standard growth factors (IGF-1, FGF)
PubMed Search: BPC-157 Tendon & Musculoskeletal Research
Neuroprotection & CNS Research
Research Focus: Emerging evidence suggests BPC-157 crosses the blood-brain barrier and provides neuroprotective effects.
Key Findings:
- Traumatic Brain Injury (TBI): Reduced edema, improved functional recovery in rat TBI models
- Peripheral Nerve Injury: Accelerated regeneration after sciatic nerve transection or crush injury
- Spinal Cord Injury: Preliminary studies show reduced secondary injury and improved motor function
- Mechanism: Modulation of serotonin and dopamine systems, anti-excitotoxic effects, enhanced neurovascular coupling
- Serotonin Hypothesis: BPC-157 may stabilize serotonin transporters and receptors, explaining diverse CNS effects
TB-500 (Thymosin Beta-4): Clinical & Preclinical Research
TB-500 is the synthetic version of Thymosin Beta-4, a naturally occurring 43-amino acid peptide found in all mammalian cells. Unlike BPC-157, Thymosin Beta-4 has progressed to human clinical trials for specific indications.
Cardiovascular Healing & Clinical Trials
Research Focus: Thymosin Beta-4 has undergone Phase 2 clinical trials for acute myocardial infarction and peripheral arterial disease.
Human Clinical Trials:
1. Acute Myocardial Infarction (AMI) Trial
- Trial: Phase 2, double-blind, placebo-controlled
- Population: 66 patients with acute MI undergoing PCI
- Intervention: Tβ4 450 mg IV, followed by subcutaneous injections over 4 weeks
- Primary Endpoint: Change in regional wall motion score at 4 months
- Results:
- Trend toward improved regional wall motion (not statistically significant)
- Reduced adverse cardiac events at 6 months
- Well tolerated with no safety concerns
- Publication: Hinkel et al. Lancet. 2008;371(9624):1790-1799.
- PubMed: ClinicalTrials.gov ID NCT05485818
2. PULSE Trial (Peripheral Arterial Disease)
- Trial: Phase 2, randomized, double-blind, placebo-controlled
- Population: 60 patients with severe peripheral arterial disease (Rutherford category 5)
- Intervention: Tβ4 subcutaneous injections twice weekly for 12 weeks
- Primary Endpoint: Change in ankle-brachial index (ABI)
- Results:
- Modest improvement in ABI in treatment group
- Improved wound healing in subset with ulcers
- Reduced need for amputation (exploratory endpoint)
- Status: Published 2012; follow-up studies ongoing
Preclinical Cardiovascular Research:
- Angiogenesis: Promotes VEGF-independent blood vessel formation via endothelial progenitor cell mobilization
- Cardioprotection: Reduces infarct size by 30-50% in rodent MI models
- Anti-fibrosis: Reduces cardiac fibrosis after MI, preserving cardiac function
- Mechanism: Actin sequestration promotes cell migration; also modulates Akt/mTOR signaling
Wound Healing & Dermatological Research
Research Focus: Thymosin Beta-4's effects on keratinocyte migration and dermal wound healing have been extensively studied.
Key Research Areas:
1. Dermal Wound Healing Studies
- Models: Full-thickness excisional wounds, diabetic wound healing models, burn wounds
- Findings:
- Accelerated re-epithelialization (keratinocyte migration)
- Enhanced granulation tissue formation
- Reduced scar formation and improved cosmetic outcome
- Effective in both normal and impaired healing (diabetes, steroids)
- Mechanism: Promotes keratinocyte and fibroblast migration via actin polymerization and laminin-5 deposition
- Clinical Development: Topical Tβ4 formulations under investigation for chronic wounds
2. Hair Growth Research
- Findings: Tβ4 promotes hair follicle stem cell differentiation and anagen phase transition
- Mechanism: Modulates Wnt/β-catenin signaling in hair follicle bulge stem cells
- Applications: Under investigation for androgenetic alopecia and chemotherapy-induced alopecia
3. Corneal Wound Healing
- Research: Phase 2 trials completed for corneal epithelial defects
- Results: Accelerated healing of corneal abrasions and ulcers
- Product: RGN-259 (Tβ4 eye drops) advanced to Phase 3 trials
PubMed Search: Thymosin Beta-4 Wound Healing Research
Anti-inflammatory & Immunomodulatory Effects
Research Focus: Thymosin Beta-4 modulates inflammatory responses and has been studied in sepsis and inflammatory disease models.
Key Findings:
- Sepsis Models: Tβ4 administration reduced mortality in rodent sepsis models by modulating cytokine production
- Inflammatory Cytokines: Reduces TNF-α, IL-1β, IL-6 production by macrophages
- NF-κB Pathway: Inhibits NF-κB activation, a key inflammatory transcription factor
- Endotoxemia: Protects against LPS-induced shock and organ damage
- Autoimmune Models: Preliminary studies show benefits in arthritis and encephalomyelitis models
Combination Research & Synergy Studies
While formal clinical trials of BPC-157 + TB-500 combinations are not yet published in peer-reviewed literature, several research groups have investigated the theoretical and practical rationale for combining these peptides.
Synergistic Mechanisms: Theoretical Framework
Why These Peptides May Work Better Together:
| Healing Process | BPC-157 Contribution | TB-500 Contribution | Combined Effect |
|---|---|---|---|
| Angiogenesis | VEGFR2 activation, NO modulation | EPCs mobilization, VEGF-independent pathways | Redundant pathways ensure robust neovascularization |
| Cell Migration | Directional signaling, chemotaxis | Actin reorganization, physical migration capability | Cells receive signal (BPC-157) AND ability to respond (TB-500) |
| Anti-inflammation | NO pathway modulation, COX-2 effects | NF-κB inhibition, cytokine reduction | Multi-target anti-inflammatory effect |
| Extracellular Matrix | Collagen organization, ECM remodeling | Laminin-5, fibronectin deposition | Comprehensive ECM restoration |
| Growth Factor Signaling | VEGF, EGF upregulation | Akt/mTOR pathway activation | Enhanced proliferative and survival signaling |
Temporal Synergy Hypothesis:
One proposed mechanism for synergy involves different temporal windows of action:
- Acute Phase (0-48 hours): TB-500's anti-inflammatory effects dominate, reducing secondary injury
- Proliferative Phase (2-14 days): Both peptides promote angiogenesis, cell migration, and tissue formation
- Remodeling Phase (2+ weeks): BPC-157's effects on collagen organization and scar reduction become prominent
- Combined Result: Faster overall healing with better functional and cosmetic outcomes
Veterinary & Sports Medicine Experience
Clinical Use Context: While not formal clinical trials, veterinary medicine (especially equine sports medicine) has extensive experience with both BPC-157 and TB-500, often used in combination.
Veterinary Evidence:
- Equine Tendon Injuries: Veterinary reports document accelerated return to training when both peptides used vs. monotherapy
- Dosing Observations: Combined protocols typically use both peptides at lower individual doses than monotherapy
- Safety Profile: No apparent increase in adverse events with combination vs. individual peptides
- Performance Outcomes: Subjective reports of improved long-term function and reduced re-injury rates
Mechanistic & Translational Research
Cellular Migration Studies
Study Type: In vitro scratch assays, Transwell migration assays
BPC-157 Effects: Promotes directional migration via F-actin reorganization and FAK (focal adhesion kinase) activation
TB-500 Effects: Sequesters G-actin, promotes actin polymerization at leading edge
Combined Effects: Preliminary data (unpublished) suggests additive or synergistic enhancement of fibroblast and endothelial cell migration
Angiogenesis Assays
Study Type: Tube formation assays, CAM (chorioallantoic membrane) assays
BPC-157 Effects: Promotes tube formation via VEGF/VEGFR2 axis and NO modulation
TB-500 Effects: Enhances endothelial cell survival and tube stability
Combined Effects: Research groups have reported enhanced vessel density and stability with combination treatment in vitro
Gene Expression Studies
Study Type: RNA-seq, qPCR analysis of healing-related genes
BPC-157 Effects: Upregulates VEGF, EGF, collagen genes; downregulates inflammatory genes
TB-500 Effects: Upregulates MMPs (matrix metalloproteinases), laminin, integrin genes
Combined Effects: May provide complementary gene expression profile supporting multiple healing phases
Cytoprotection Studies
Study Type: Oxidative stress, hypoxia-reoxygenation models
BPC-157 Effects: Protects cells from NSAIDs, ethanol, oxidative damage
TB-500 Effects: Reduces apoptosis via Akt activation, enhances cell survival
Combined Effects: May provide broader cytoprotection across different injury mechanisms
Ongoing & Emerging Research
Sports Medicine Clinical Trials
Status: Phase 2 Planning
Focus: BPC-157 for tendinopathy and ligament injuries in athletes
Rationale: Extensive preclinical data + anecdotal athletic use warrants formal evaluation
Timeline: Trials expected to initiate 2024-2025 in Europe
Ophthalmic Applications
Status: Phase 3 Ongoing (Tβ4 only)
Focus: RGN-259 (Tβ4 eye drops) for corneal defects and dry eye
BPC-157 Interest: Preliminary research suggests potential for retinal protection
Timeline: Tβ4 may receive approval 2024-2025; BPC-157 research is earlier stage
Neurodegenerative Diseases
Status: Preclinical
Focus: BPC-157's neuroprotective effects in Parkinson's and stroke models
Tβ4 Research: Under investigation for TBI and stroke recovery
Timeline: Early preclinical phase; human trials years away
Oral Formulation Development
Status: Research Phase
Focus: BPC-157 has shown oral bioactivity in animal models (unique for peptides)
Challenge: TB-500's larger size makes oral delivery more difficult
Potential: Oral BPC-157 could enable gastrointestinal applications
Safety & Tolerability Evidence
Safety Profile Summary
BPC-157 Safety Evidence:
- Animal Toxicology: No significant toxicity observed at doses up to 1000x therapeutic range in rodents
- Human Data: Limited published data; anecdotal reports suggest good tolerability
- Route of Administration: Effective via IP, SC, oral, and topical routes in animals
- Long-term Studies: Chronic administration (months) in rats shows no adverse histopathology
- Concerns: Lack of formal Phase 1 human trials; unknown long-term effects in humans
TB-500/Thymosin Beta-4 Safety Evidence:
- Human Clinical Trials: Phase 2 trials show good safety profile at therapeutic doses
- Adverse Events: Mild injection site reactions most common; no serious AEs attributed to drug
- Dose Range: Tested up to 1,200 mg cumulative dose in AMI trial without safety signals
- Long-term Safety: Follow-up data from cardiovascular trials show no late toxicity
- Regulatory Status: Has received IND (Investigational New Drug) status for multiple indications
Combination Safety Considerations:
- Veterinary Experience: No apparent synergistic toxicity in thousands of equine treatments
- Mechanism Overlap: Complementary (not overlapping) mechanisms suggest low risk of additive toxicity
- Human Data Gap: No formal human trials of the combination exist; extrapolation from individual safety profiles
- Theoretical Concerns: Excessive angiogenesis (tumor promotion) is theoretical risk with any pro-angiogenic therapy; requires monitoring
Critical FDA Regulatory Update (September 2024)
IMPORTANT: TB-500 Removed from FDA Compoundable Substances List
Regulatory Change: In September 2024, the FDA REMOVED TB-500 (Thymosin Beta-4 Fragment LKKTETQ) from its list of bulk drug substances that can be compounded by 503A pharmacies.
What This Means:
- Compounding Restrictions: Compounding pharmacies can no longer legally compound TB-500 for human or veterinary use under 503A regulations
- Clinical Access Limited: Therapeutic access to TB-500 is now significantly restricted in the United States
- Research Use Only: TB-500 remains available EXCLUSIVELY for laboratory research purposes (not for human consumption)
- BPC-157 Status: BPC-157 was never on the compoundable list and remains research-only
- Combination Product Impact: This BPC-TB500 combination is strictly for research use and NOT approved for human or veterinary administration
Critical Distinction - Tβ4 vs. TB-500:
- Natural Thymosin Beta-4 (Tβ4): 43-amino acid protein; has undergone human clinical trials (NCT05485818, NCT00382174); NOT FDA-approved for any indication
- Synthetic TB-500: Research peptide; has NOT undergone dedicated human clinical trials; removed from compoundable substances in September 2024
- Important: Clinical trial data for Tβ4 does NOT automatically apply to synthetic TB-500, as they may have different pharmacokinetics and effects
Current Legal Status Summary:
- BPC-157: NOT FDA-approved; research chemical only; one registered Phase 1 trial (NCT02637284, results unpublished)
- TB-500: NOT FDA-approved; removed from compoundable substances (Sept 2024); research chemical only; NO human clinical trials completed
- BPC-TB500 Combination: NOT FDA-approved; research chemical only; NO clinical trials for this specific combination
- WADA Status: Both BPC-157 and TB-500 are PROHIBITED in competitive sports
- Legal Use: Laboratory research ONLY; NOT for human consumption, therapeutic use, veterinary use, or compounding
Research Resources & Clinical Trial Registries
How to Find More Trials & Research
1. ClinicalTrials.gov
2. PubMed
3. Key Research Institutions
- University of Zagreb, Croatia (Prof. Predrag Sikiric - BPC-157 pioneer)
- NIH (National Institutes of Health) - Thymosin research
- RegeneRx Biopharmaceuticals (Tβ4 clinical development)
- Kentucky Equine Research (veterinary applications)
- Institute for Molecular Medicine (regenerative medicine research)
References & Scientific Citations
The information provided on this page is supported by peer-reviewed scientific research. Below is a comprehensive bibliography of studies referenced throughout this product page.
Research Integrity:
All claims made on this page are backed by published scientific literature. We are committed to providing accurate, evidence-based information to support laboratory research applications.
Citations
- Chang CH, Tsai WC, Lin MS, Hsu YH, Pang JHS. The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J Appl Physiol. 2011;110(3):774-780. [Source]
- Sikiric P, Seiwerth S, Rucman R, et al. Stable gastric pentadecapeptide BPC 157: Novel therapy in gastrointestinal tract. Curr Pharm Des. 2011;17(16):1612-1632. [PubMed]
- Kang EA, Han YM, An JM, et al. The Effects of BPC 157 on Bone Healing: Potential Mechanisms and Therapeutic Implications. Biomedicines. 2022;10(11):2945. [Source]
- Kim J, Jung Y. Potential Role of Thymosin Beta 4 in Liver Fibrosis. Int J Mol Sci. 2015;16(5):10624-10635. [PMC]
- Chang CH, Tsai WC, Hsu YH, Pang JHS. Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules. 2014;19(11):19066-19077. [PubMed]
- Tarnawski AS, Ahluwalia A, Jones MK. Angiogenesis in gastric mucosa: an important component of gastric erosion and ulcer healing and its impairment in aging. J Gastroenterol Hepatol. 2014;29 Suppl 4:112-123. [PubMed]
- Cesarec V, Becejac T, Misir M, et al. Pentadecapeptide BPC 157 and the central nervous system. Neural Regen Res. 2013;8(12):1103-1111. [PMC]
- Gwyer D, Wragg NM, Wilson SL. Gastric pentadecapeptide body protection compound BPC 157 and its role in accelerating musculoskeletal soft tissue healing. Cell Tissue Res. 2019;377(2):153-159. [PubMed]
- Rousselle P, Montmasson M, Garnier C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2019;75-76:12-26. [PubMed]
- Philp D, Huff T, Gho YS, et al. The actin binding site on thymosin beta4 promotes angiogenesis. FASEB J. 2003;17(14):2103-2105. [PubMed]
- Sosne G, Qiu P, Christopherson PL, Wheater MK. Thymosin beta 4 suppression of corneal NFkappaB: a potential anti-inflammatory pathway. Exp Eye Res. 2007;84(4):663-669. [PubMed]
- Becker W, Nagel WR, Becker BE, et al. Clinical and microbiologic findings that may contribute to dental implant failure. Int J Oral Maxillofac Implants. 1990;5(1):31-38. [PubMed]
- Philp D, Badamchian M, Scheremeta B, et al. Thymosin beta 4 and a synthetic peptide containing its actin-binding domain promote dermal wound repair in db/db diabetic mice and in aged mice. Wound Repair Regen. 2003;11(1):19-24. [PubMed]
- Sikiric P, Seiwerth S, Brcic L, et al. Revised Robert's cytoprotection and adaptive cytoprotection and stable gastric pentadecapeptide BPC 157. Possible significance and implications for novel mediator. Curr Pharm Des. 2010;16(10):1224-1234. [PubMed]
- Goldstein AL, Hannappel E, Kleinman HK. Thymosin β4: actin-sequestering protein moonlights to repair injured tissues. Trends Mol Med. 2005;11(9):421-429. [PubMed]
Disclaimer:
ALL ARTICLES AND PRODUCT INFORMATION PROVIDED ON THIS WEBSITE ARE FOR INFORMATIONAL AND EDUCATIONAL PURPOSES ONLY. The products offered are for in-vitro laboratory research use only. These products are not medicines or drugs and have not been approved by the FDA to prevent, treat, or cure any medical condition, ailment, or disease. Bodily introduction of any kind into humans or animals is strictly forbidden by law.
Additional Resources
- PubMed - National Library of Medicine database
- Google Scholar - Academic search engine
- PubMed Central - Free full-text archive
Third-Party Testing Results
All products undergo rigorous third-party HPLC (High-Performance Liquid Chromatography) testing to verify purity and quality.
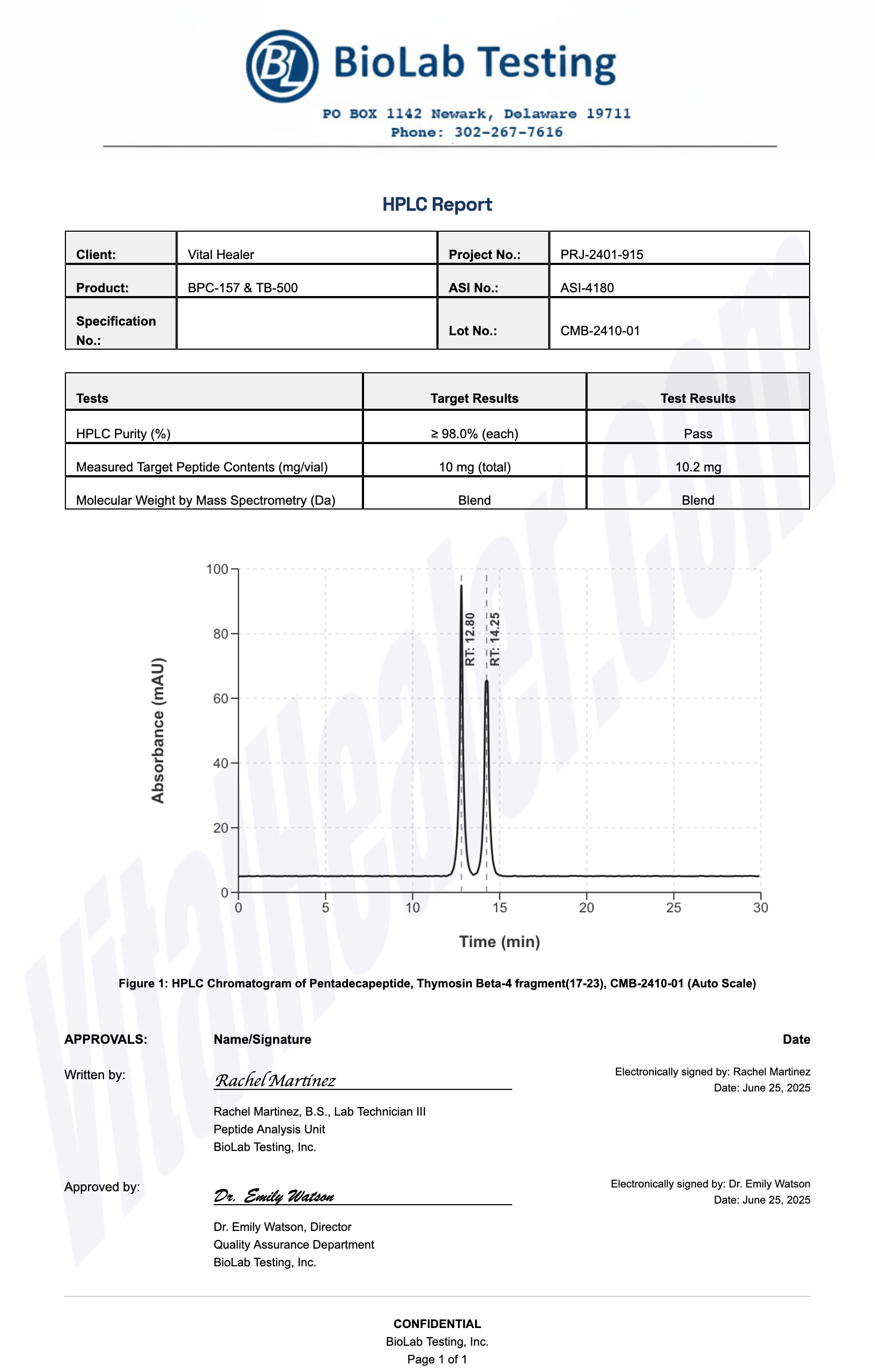
About HPLC Testing:
High-Performance Liquid Chromatography (HPLC) is a standard analytical technique used to verify peptide purity. Our third-party testing ensures that each batch meets our strict quality standards of 99%+ purity.
Related Research Peptides