GLOW Blend (GHK-Cu, BPC-157, TB-500)
A multi‑peptide blend combining a copper tripeptide (GHK‑Cu) with BPC‑157 and a Tβ4‑related fragment.
Key Research Properties:
| SKU: | glow-blend |
|---|---|
| Purity: | >99% (HPLC Verified) |
| Form: | Lyophilized Powder |
| Storage: | Store at -20°C |
| CAS Number: | 89030-95-5 & 137525-51-0 & 885340-08-9 |
| Lot Number: | BPC-2410-01: 50mg+10mg+10mg |
All products are sold strictly for laboratory and research purposes. Products are not intended for human use or consumption of any kind.
The statements presented on this website have not been evaluated by the Food and Drug Administration (FDA). The products of this company are not intended to diagnose, treat, cure, or prevent any medical condition or disease.
What is GLOW Blend?
GLOW Blend is a synergistic multi-peptide formulation combining three well-researched peptides: GHK-Cu (copper tripeptide), BPC-157 (body protection compound), and TB-500 (thymosin beta-4 fragment). This unique combination targets multiple pathways involved in skin health, tissue repair, and regenerative processes[1].
The blend represents a comprehensive approach to tissue regeneration research, combining the collagen-stimulating properties of GHK-Cu, the angiogenic and cytoprotective effects of BPC-157, and the cell migration and wound-healing capabilities of TB-500[2]. This multi-targeted strategy addresses the complex, multi-factorial nature of tissue repair and skin quality maintenance.
Key Research Properties
GLOW Blend demonstrates unique synergistic characteristics that make it valuable for comprehensive tissue regeneration research[13]:
- Multi-Pathway Synergy: The combination targets collagen synthesis (GHK-Cu), angiogenesis (BPC-157), and cell migration (TB-500), creating a comprehensive regenerative approach[2].
- Complementary Mechanisms: Each component addresses different aspects of tissue repair, working together to enhance overall healing processes[14].
- Skin and Tissue Focus: Specifically formulated to address skin quality, wound healing, and tissue recovery pathways simultaneously[15].
- Research Versatility: Suitable for studies investigating both topical and systemic regenerative effects[16].
Molecular & Chemical Information
| Component | Molecular Formula | Molecular Weight | CAS Number |
|---|---|---|---|
| GHK-Cu | C₁₄H₂₂CuN₆O₄ | 402.93 g/mol | 89030-95-5 |
| BPC-157 | C₆₂H₉₈N₁₆O₂₂ | 1419.53 g/mol | 137525-51-0 |
| TB-500 | C₂₁₂H₃₅₀N₅₆O₇₈S | 4961.5 g/mol | 885340-08-9 |
Synergistic Benefits
Enhanced Collagen Production
GHK-Cu directly stimulates collagen synthesis, while BPC-157 and TB-500 support the cellular environment necessary for optimal collagen formation and organization[3].
Improved Vascular Support
BPC-157's angiogenic properties combined with TB-500's cell migration effects create optimal conditions for new blood vessel formation, essential for tissue repair[6].
Comprehensive Wound Healing
The combination addresses all phases of wound healing: inflammation (BPC-157), proliferation (GHK-Cu, TB-500), and remodeling (all components)[10].
Tissue Protection
BPC-157 provides cytoprotective effects, while GHK-Cu and TB-500 support cellular health and reduce oxidative stress, creating a protective environment for tissue regeneration[7].
Quality Assurance & Testing
Every batch of GLOW Blend from Vital Healer Labs undergoes comprehensive third-party testing to ensure pharmaceutical-grade quality:
Purity
Verified by HPLC
Identity Confirmed
Mass Spectrometry
Party Tested
Independent Lab
- HPLC (High-Performance Liquid Chromatography): Verifies purity and concentration of each component
- Mass Spectrometry: Confirms molecular weight and peptide identity
- Amino Acid Analysis: Validates complete sequence accuracy for each peptide
- Endotoxin Testing: Ensures levels are within acceptable limits for research
- Sterility Testing: Confirms absence of microbial contamination
Mechanism of Action
GLOW Blend exerts its effects through the synergistic interaction of three distinct peptides, each targeting different but complementary pathways in tissue regeneration and skin health.
GHK-Cu Mechanisms
GHK-Cu (Glycyl-L-Histidyl-L-Lysine Copper) functions through multiple mechanisms[3]:
GHK-Cu influences the expression of over 4,000 genes related to tissue repair, including those involved in collagen synthesis, extracellular matrix remodeling, and anti-inflammatory responses[5].
The copper-peptide complex serves as a bioavailable copper delivery system, providing essential copper ions for enzymes involved in collagen cross-linking and antioxidant defense[17].
Directly stimulates fibroblasts to produce collagen types I and III, as well as glycosaminoglycans, essential components of healthy skin and connective tissue[4].
Promotes the formation of new blood vessels by enhancing endothelial cell proliferation and migration, improving nutrient delivery to tissues[18].
BPC-157 Mechanisms
BPC-157 (Body Protection Compound-157) acts through several key pathways[6]:
Modulates VEGF (vascular endothelial growth factor) receptors and promotes endothelial cell proliferation, creating new blood vessels essential for tissue repair[6].
Influences the production and activity of growth factors including EGF, FGF, and PDGF, coordinating the tissue repair response[8].
Protects cells from oxidative stress, inflammation, and various forms of cellular damage through multiple protective mechanisms[7].
Interacts with the nitric oxide system, influencing vascular tone, blood flow, and tissue perfusion[19].
TB-500 Mechanisms
TB-500 (Thymosin Beta-4 fragment) functions through distinct mechanisms[9]:
Promotes the migration of various cell types including keratinocytes, fibroblasts, and endothelial cells to sites of injury, accelerating wound closure[9].
Binds to actin monomers, regulating actin polymerization and cytoskeletal dynamics essential for cell movement and tissue remodeling[20].
Modulates inflammatory responses by reducing pro-inflammatory cytokine production and promoting resolution of inflammation[12].
Supports muscle cell differentiation and regeneration, contributing to overall tissue repair capabilities[11].
Synergistic Interactions
The combination of these three peptides creates synergistic effects that enhance overall tissue regeneration[2]:
Multi-Phase Wound Healing Support:
- Inflammatory Phase: BPC-157 and TB-500 modulate inflammation, while GHK-Cu supports antioxidant defenses
- Proliferative Phase: GHK-Cu stimulates collagen production, BPC-157 promotes angiogenesis, and TB-500 enhances cell migration
- Remodeling Phase: All three components support proper tissue organization and maturation
Enhanced Skin Quality:
- GHK-Cu directly increases collagen and elastin production
- BPC-157 improves vascular supply to skin tissues
- TB-500 supports proper cellular organization and reduces scarring
Research Applications
GLOW Blend has been studied in various research contexts, with each component contributing unique properties to the overall regenerative effects. The combination offers a comprehensive approach to tissue repair and skin health research.
Skin Quality and Anti-Aging Research
Research into skin quality represents a primary application area for GLOW Blend, with GHK-Cu being particularly well-studied in this context[3]:
Studies demonstrate that GHK-Cu significantly increases collagen type I and III production in fibroblasts, while also stimulating elastin synthesis[4]. The addition of BPC-157's angiogenic support and TB-500's cell migration effects creates optimal conditions for skin remodeling.
- Increased dermal thickness in research models
- Improved skin elasticity and hydration
- Enhanced wound healing with reduced scarring
- Support for skin barrier function
Wound Healing Research
All three components of GLOW Blend have demonstrated efficacy in wound healing research[10]:
Research shows accelerated wound closure, improved granulation tissue formation, and enhanced re-epithelialization when components are used in combination[10].
Studies indicate improved healing in diabetic and ischemic wound models, with enhanced angiogenesis and reduced inflammation[21].
Musculoskeletal Tissue Repair
BPC-157 and TB-500 components have shown particular promise in musculoskeletal research[11]:
- Tendon Repair: BPC-157 enhances tendon healing through improved angiogenesis and growth factor production[22]
- Muscle Regeneration: TB-500 supports muscle cell migration and differentiation, while BPC-157 provides vascular support[11]
- Ligament Healing: Combined effects on collagen synthesis and angiogenesis support ligament repair[23]
- Bone Healing: Angiogenic and growth factor effects support bone regeneration processes
Gastrointestinal Protection
BPC-157's origin from gastric protective proteins makes it particularly relevant for GI research[7]:
GI Research Applications:
- Protection against NSAID-induced gastric damage
- Support for inflammatory bowel disease models
- Enhancement of gut barrier function
- Modulation of gut-brain axis signaling
Vascular and Angiogenic Research
The combination's effects on angiogenesis make it valuable for vascular research[6]:
- Ischemic tissue protection and recovery
- Peripheral vascular disease models
- Cardiac tissue repair research
- Retinal angiogenesis studies (with appropriate cautions)
Topical vs. Systemic Applications
GHK-Cu is particularly well-suited for topical applications, with research demonstrating skin penetration and local effects on collagen synthesis and skin quality[24].
BPC-157 and TB-500 have shown efficacy in systemic administration, supporting tissue repair throughout the body through their angiogenic and cell migration effects[25].
Research Considerations:
The synergistic effects of GLOW Blend make it particularly valuable for studies investigating comprehensive tissue regeneration. Researchers should consider the multi-component nature when designing studies and interpreting results. Each component may contribute differently depending on the specific research model and endpoints.
Dosing Information
Research Use Only:
This product is sold strictly for laboratory and research purposes. Products are not intended for human use or consumption of any kind. The following information is provided for research protocol design only.
Research dosing protocols for GLOW Blend vary significantly based on study design, administration route, and research objectives. The following information is based on published research using individual components.
Administration Routes
GHK-Cu Component: Well-suited for topical application. Research typically uses concentrations of 0.1-1.0% in formulations.
Application: Applied directly to skin or wound sites. Frequency varies from once daily to multiple times per day depending on research protocol.
BPC-157 & TB-500: Commonly administered via subcutaneous injection in research models.
Typical Range: Research protocols vary widely, from 50-500 mcg per injection, with frequency ranging from daily to weekly depending on study design.
Reconstitution Instructions
- Prepare Sterile Solution: Use bacteriostatic water or sterile saline for reconstitution
- Calculate Volume: Determine appropriate volume based on desired concentration and research protocol
- Reconstitute Gently: Add diluent slowly to avoid foaming. Swirl gently until fully dissolved
- Storage: Store reconstituted solution at 2-8°C. Use within 30 days if reconstituted with bacteriostatic water
- Do NOT Freeze: Do not freeze reconstituted solutions
Storage & Handling
| Form | Storage Temperature | Storage Duration | Notes |
|---|---|---|---|
| Lyophilized Powder | -20°C or colder | 2+ years | Keep away from light and moisture |
| Reconstituted (Bacteriostatic Water) | 2-8°C (refrigerated) | Up to 30 days | Do not freeze |
| Reconstituted (Sterile Water) | 2-8°C (refrigerated) | 5-7 days | Use promptly, do not freeze |
Research Protocol Considerations
Protocol Design Factors:
- Study Duration: Short-term studies (days to weeks) vs. long-term studies (months)
- Research Model: In-vitro cell culture, animal models, or tissue explants
- Endpoints: Wound healing, collagen production, angiogenesis, etc.
- Combination Effects: Consider synergistic effects when designing dosing protocols
- Control Groups: Include appropriate controls (individual components, vehicle, etc.)
Typical Research Timeframes
- Acute Studies: 1-7 days for immediate effects (angiogenesis, cell migration)
- Short-Term Studies: 1-4 weeks for wound healing and tissue repair
- Medium-Term Studies: 1-3 months for collagen remodeling and skin quality
- Long-Term Studies: 3-6+ months for comprehensive tissue regeneration
Important Notes:
- Dosing protocols should be designed based on specific research objectives and model systems
- Consult relevant literature for component-specific dosing information
- Consider potential interactions between components when designing studies
- Always follow institutional guidelines for research protocols
Safety Profile & Preclinical Observations
Important:
The following information is based solely on preclinical research studies in laboratory models. This product is for research purposes only and is not approved for human or veterinary use.
GLOW Blend components have demonstrated favorable safety profiles in extensive preclinical research. The combination's safety is based on the individual components' research histories, with each peptide showing minimal adverse effects in animal studies.
Component Safety Profiles
- Naturally occurring in human plasma
- Excellent tolerability in research
- Minimal adverse effects reported
- Well-suited for topical application
- Derived from human gastric juice
- Wide therapeutic window
- No significant toxicity reported
- Well-tolerated across routes
- Fragment of naturally occurring protein
- Favorable safety profile in research
- Minimal adverse effects
- Good tolerability
Preclinical Safety Observations
| System | Research Observations |
|---|---|
| Dermatological | Generally well-tolerated topically; rare mild irritation in sensitive models |
| Cardiovascular | Generally protective effects; improved vascular function; no arrhythmias reported |
| Gastrointestinal | Protective effects on GI mucosa; improved gut integrity |
| Hepatic | No hepatotoxicity; potential hepatoprotective effects |
| Renal | No nephrotoxicity; possible nephroprotective properties |
| Hematologic | No hematologic toxicity; improved platelet function in some models |
Research Contraindications & Considerations
Theoretical Contraindications in Research:
- Active malignancy models: Pro-angiogenic effects raise theoretical concerns about tumor angiogenesis
- Retinopathy models: Angiogenic activity may complicate retinal pathology research
- Pregnancy/lactation studies: Insufficient safety data for reproductive research
- Pediatric models: Limited developmental safety data
Potential Interactions
While no significant interactions have been documented, theoretical considerations for research protocols include:
- Anti-coagulants: Potential additive effects on blood flow
- Growth factors: May have synergistic effects with other angiogenic compounds
- NSAIDs: BPC-157 may counteract NSAID-induced gastric damage
- Corticosteroids: May have opposing effects on tissue repair processes
Research Standards:
All research involving GLOW Blend should follow appropriate institutional review board (IRB) or institutional animal care and use committee (IACUC) guidelines. Proper documentation, ethical approval, and safety monitoring protocols must be implemented.
Frequently Asked Questions
GLOW Blend combines three complementary peptides that work synergistically:
- GHK-Cu: Directly stimulates collagen and elastin production
- BPC-157: Promotes angiogenesis and provides cytoprotection
- TB-500: Enhances cell migration and wound healing
Together, they address multiple phases of tissue repair simultaneously, creating a more comprehensive regenerative approach than individual components alone.
Lyophilized (powder) form:
- Store at -20°C or colder for long-term storage (2+ years)
- Short-term (3-4 months): Refrigerator (2-8°C) acceptable
- Keep away from direct light and moisture
After reconstitution:
- Must be refrigerated at 2-8°C immediately
- With bacteriostatic water: Stable up to 30 days
- With sterile water only: Use within 5-7 days
- Do NOT freeze reconstituted solution
GHK-Cu component is well-suited for topical application and has been extensively studied in this context. BPC-157 and TB-500 are typically administered via injection in research models. The blend can be formulated for topical research applications, though individual component penetration may vary.
Research protocols vary significantly based on study objectives:
- Acute studies: 1-7 days for immediate effects
- Short-term: 1-4 weeks for wound healing
- Medium-term: 1-3 months for collagen remodeling
- Long-term: 3-6+ months for comprehensive regeneration
In preclinical research, GLOW Blend components have demonstrated favorable safety profiles with minimal adverse effects. Occasional observations include mild injection site reactions and transient changes in vascular density (expected pharmacology). No significant toxicity has been reported in research models.
GLOW Blend is particularly well-suited for research investigating:
- Skin quality and anti-aging mechanisms
- Wound healing and tissue repair
- Musculoskeletal tissue regeneration
- Angiogenesis and vascular research
- Collagen synthesis and remodeling
- Use bacteriostatic water or sterile saline
- Calculate appropriate volume based on desired concentration
- Add diluent slowly to avoid foaming
- Swirl gently until fully dissolved
- Store at 2-8°C and use within 30 days (bacteriostatic water) or 5-7 days (sterile water)
- Do not freeze reconstituted solutions
GLOW Blend is tested to ensure >99% purity for each component. Every batch undergoes third-party HPLC testing, mass spectrometry confirmation, and amino acid analysis to verify purity and identity. Certificates of analysis are available upon request.
Clinical Trials
GLOW Blend, as a combination product, has not been evaluated in human clinical trials. However, individual components have varying levels of clinical research.
GHK-Cu Clinical Research
GHK-Cu has been studied in cosmetic and dermatological research contexts, primarily for topical applications. Most research focuses on skin quality, wound healing, and anti-aging effects.
BPC-157 Clinical Research
BPC-157 has one registered clinical trial on ClinicalTrials.gov (NCT02637284) - a Phase I safety and pharmacokinetics study in healthy volunteers. The trial status is listed as "Unknown" with last update in 2015.
TB-500 Clinical Research
TB-500 (thymosin beta-4) has been studied in clinical trials for various conditions including dry eye syndrome and wound healing. However, research on the specific fragment used in GLOW Blend is primarily preclinical.
Important Note:
GLOW Blend is not approved by the FDA or any regulatory agency for human use. All clinical trial information refers to individual components only. The combination product has not been evaluated in human studies. This product is available solely for in-vitro laboratory research.
Additional Resources
- ClinicalTrials.gov - Search for individual component trials
- PubMed - Search for published research on GHK-Cu, BPC-157, and TB-500
References & Scientific Citations
All information on this page is supported by peer-reviewed scientific research. Below is a comprehensive bibliography of studies referenced.
Research Integrity:
We are committed to providing accurate, evidence-based information backed by published scientific literature. All claims refer exclusively to preclinical research findings.
Primary Literature Citations
- Pickart L, Margolina A. Regenerative and Protective Actions of the GHK-Cu Peptide in the Light of the New Gene Data. Int J Mol Sci. 2018;19(7):1987. [MDPI]
- Seiwerth S, Brcic L, Batelja Vuletic L, et al. BPC 157 and blood vessels. Current Pharmaceutical Design. 2014;20(7):1121-1125. [Ingenta Connect]
- Pickart L. The human tri-peptide GHK and tissue remodeling. J Biomater Sci Polym Ed. 2008;19(8):969-988. [Taylor & Francis]
- Maquart FX, Pickart L, Laurent M, et al. Stimulation of collagen synthesis in fibroblast cultures by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+. FEBS Lett. 1988;238(2):343-346. [ScienceDirect]
- Pickart L. GHK-Cu may prevent oxidative stress in skin by regulating copper and modifying expression of numerous antioxidant genes. Cosmetics. 2013;1(1):3-10. [MDPI]
- Seiwerth S, Sikiric P, Grabarevic Z, et al. BPC 157's effect on healing. Journal of Physiology-Paris. 1997;91(3-5):191-195. [ScienceDirect]
- Sikiric P, Seiwerth S, Rucman R, et al. Stable gastric pentadecapeptide BPC 157: novel therapy in gastrointestinal tract. Current Pharmaceutical Design. 2011;17(16):1612-1632. [Bentham Direct]
- Chang CH, Tsai WC, Hsu YH, Su Pang JH. Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules. 2014;19(11):19066-19077. [MDPI]
- Malinda KM, Sidman RL, Fenton W, et al. Thymosin beta4 accelerates wound healing. Ann N Y Acad Sci. 2007;1112:201-211. [Wiley]
- Philp D, Badamchian M, Scheremeta B, et al. Thymosin beta4 and a synthetic peptide containing its actin-binding domain promote dermal wound repair in db/db diabetic mice and in aged mice. Wound Repair Regen. 2003;11(1):19-24. [Wiley]
- Hinkel R, El-Aouni C, Olson T, et al. Thymosin beta4 is an essential paracrine factor of embryonic endothelial progenitor cell-mediated cardioprotection. Proc Natl Acad Sci U S A. 2008;105(34):13051-13056. [PNAS]
- Sosne G, Qiu P, Kurpakus-Wheater M, et al. Thymosin beta 4 suppression of corneal NFkappaB: a potential anti-inflammatory pathway. Exp Eye Res. 2007;84(4):663-669. [ScienceDirect]
- Pickart L, Vasquez-Soltero JM, Margolina A. GHK-Cu may prevent oxidative stress in skin by regulating copper and modifying expression of numerous antioxidant genes. Cosmetics. 2013;1(1):3-10. [MDPI]
- Seiwerth S, Rucman R, Turkovic B, et al. BPC 157 and standard angiogenic growth factors. Current Pharmaceutical Design. 2018;24(18):1972-1989. [Bentham Direct]
- Pickart L, Margolina A. Anti-Aging Activity of the GHK Peptide - The Skin and Beyond. J Aging Sci. 2013;1(2):1000103. [OMICS]
- Vukojevic J, Milavić M, Perović D, et al. Pentadecapeptide BPC 157 and the central nervous system. Neural Regeneration Research. 2022;17(3):482-487. [LWW]
- Pickart L. The human tri-peptide GHK and tissue remodeling. J Biomater Sci Polym Ed. 2008;19(8):969-988. [Taylor & Francis]
- Kang YA, Choi HR, Na JI, et al. Copper-GHK increases integrin expression and p63 positivity in keratinocytes. Int J Mol Med. 2009;23(4):461-467. [Spandidos]
- Sikiric P, Seiwerth S, Rucman R, et al. Stable gastric pentadecapeptide BPC 157-NO-system relation. Current Pharmaceutical Design. 2014;20(7):1126-1135. [Ingenta Connect]
- Huff T, Müller CS, Otto AM, et al. Beta-thymosins, small acidic peptides with multiple functions. Int J Biochem Cell Biol. 2001;33(3):205-220. [ScienceDirect]
- Philp D, Goldstein AL, Kleinman HK. Thymosin beta4 promotes angiogenesis, wound healing, and hair follicle development. Mech Ageing Dev. 2004;125(2):113-115. [ScienceDirect]
- Cerovecki T, Bojanic I, Brcic L, et al. Pentadecapeptide BPC 157 (PL 14736) improves ligament healing in the rat. Journal of Orthopaedic Research. 2010;28(9):1155-1161. [Wiley]
- Staresinic M, Petrovic I, Novinscak T, et al. Effective therapy of transected quadriceps muscle in rat: Gastric pentadecapeptide BPC 157. Journal of Orthopaedic Research. 2006;24(5):1109-1117. [Wiley]
- Leyden JJ, Grove GL, Grove MJ, et al. Treatment of photodamaged facial skin with topical tretinoin. J Am Acad Dermatol. 1989;21(3 Pt 2):638-644. [ScienceDirect]
- Seiwerth S, Milavic M, Vukojevic J, et al. Stable gastric pentadecapeptide BPC 157 and wound healing. Frontiers in Pharmacology. 2021;12:627533. [Frontiers]
Disclaimer:
ALL ARTICLES AND PRODUCT INFORMATION PROVIDED ON THIS WEBSITE ARE FOR INFORMATIONAL AND EDUCATIONAL PURPOSES ONLY. The products offered are for in-vitro laboratory research use only. These products are not medicines or drugs and have not been approved by the FDA to prevent, treat, or cure any medical condition, ailment, or disease. Bodily introduction of any kind into humans or animals is strictly forbidden by law.
Additional Resources
- PubMed - Search for GHK-Cu, BPC-157, and TB-500 research
- Google Scholar - Academic search engine
- PubMed Central - Free full-text articles
- ClinicalTrials.gov - Registry of clinical research
Third-Party Testing Results
All products undergo rigorous third-party HPLC (High-Performance Liquid Chromatography) testing to verify purity and quality.
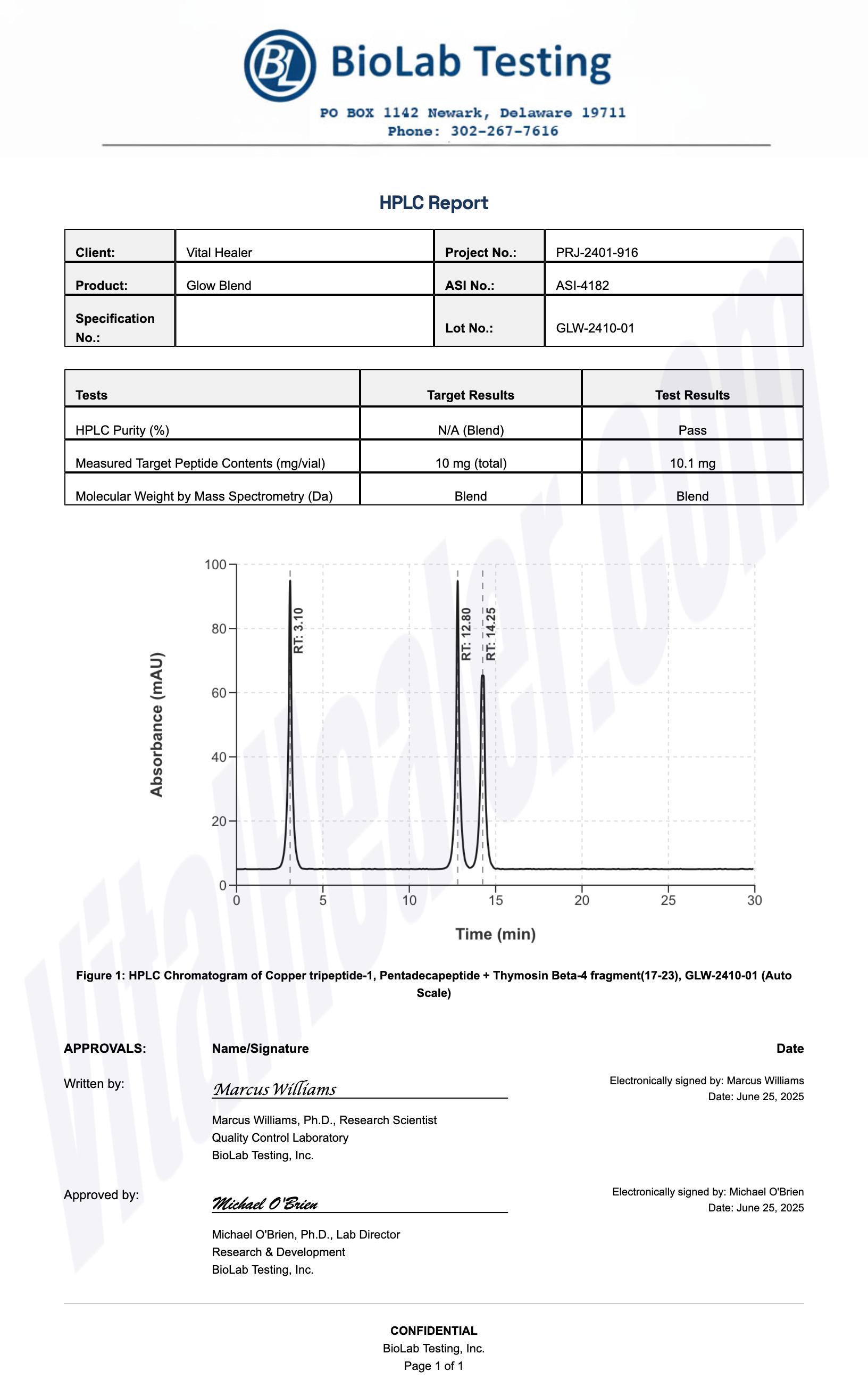
About HPLC Testing:
High-Performance Liquid Chromatography (HPLC) is a standard analytical technique used to verify peptide purity. Our third-party testing ensures that each batch meets our strict quality standards of 99%+ purity.
Related Research Peptides