Semax
A GLP‑1 receptor agonist originally developed for glycemic control that also reduces appetite.
Key Research Properties:
| SKU: | semax |
|---|---|
| Purity: | >99% (HPLC Verified) |
| Form: | Lyophilized Powder |
| Storage: | Store at -20°C |
| CAS Number: | 910463-68-2 |
| Lot Number: | SEM-2410-19: 20mg, 30mg |
All products are sold strictly for laboratory and research purposes. Products are not intended for human use or consumption of any kind.
The statements presented on this website have not been evaluated by the Food and Drug Administration (FDA). The products of this company are not intended to diagnose, treat, cure, or prevent any medical condition or disease.
What is Semax?
Semax is a synthetic heptapeptide (7-amino acid) nootropic derived from the adrenocorticotropic hormone (ACTH) fragment ACTH(4-10)[1]. Developed in Russia in the 1980s, Semax has demonstrated neuroprotective, cognitive-enhancing, and anxiolytic properties, and is approved as a pharmaceutical drug in Russia and several former Soviet states for treating stroke, cognitive decline, anxiety, and attention disorders[2].
Biochemical Properties
- Sequence: Met-Glu-His-Phe-Pro-Gly-Pro
- Molecular Weight: ~813 Da
- Derivation: Based on ACTH(4-10) with Met and Pro additions for stability
- Administration: Intranasal (primary); also IV, SC
- BBB Penetration: Yes; crosses blood-brain barrier efficiently (intranasal delivery)
- Half-life: ~1 hour (plasma); CNS effects last 6-24 hours
Primary Functions
- Neuroprotection: Protects neurons from hypoxia, oxidative stress, excitotoxicity
- Cognitive Enhancement: Improves memory, learning, attention, mental clarity
- BDNF Elevation: Increases brain-derived neurotrophic factor (neuroplasticity)
- Stroke Recovery: Reduces infarct size; accelerates neurological recovery
- Anxiolytic: Reduces anxiety without sedation; improves stress adaptation
Discovery & Development
Semax was developed in the 1980s at the Institute of Molecular Genetics, Russian Academy of Sciences, by a team led by Prof. Ashmarin[3]. The goal was to create a stable, CNS-active peptide based on ACTH fragments with neuroprotective properties but without hormonal (glucocorticoid) effects. Semax (ACTH 4-10 analog) proved more stable than native ACTH and exhibited potent nootropic effects.
Key Milestones:
- 1982-1985: Synthesis and initial preclinical studies at Russian Academy of Sciences
- 1987-1995: Clinical trials in stroke, cognitive decline, anxiety in Russia
- 1996: Approved in Russia as pharmaceutical drug (Semax®) for CNS disorders
- 2000s: Expanded research: BDNF modulation, neurogenesis, dopaminergic effects
- 2010s-present: International research interest; nootropic community adoption
Mechanism of Action
Semax exerts neuroprotective and cognitive-enhancing effects through multiple mechanisms: BDNF upregulation, modulation of monoaminergic systems (dopamine, serotonin), antioxidant effects, and anti-excitotoxic properties[4].
BDNF & Neuroplasticity
Brain-Derived Neurotrophic Factor Elevation
Primary Mechanism: Semax significantly increases BDNF expression in hippocampus, cortex, and other brain regions.
- BDNF Role: Master regulator of neuroplasticity, neurogenesis, synaptic strength, learning/memory
- Semax Effect: 1.5-3× BDNF increase in animal studies; sustained elevation 6-24 hours post-administration
- Gene Expression: Upregulates TrkB receptor (BDNF receptor); enhances downstream CREB signaling
- Neurogenesis: Promotes hippocampal neurogenesis (new neuron formation)
- Clinical Correlation: BDNF elevation explains improved learning, memory, and cognitive recovery post-stroke
Dopaminergic & Serotonergic Modulation
Monoamine Neurotransmitter Effects
Semax modulates dopamine and serotonin systems, contributing to improved mood, motivation, and attention:
- Dopamine: Increases dopamine turnover; enhances dopaminergic neurotransmission in prefrontal cortex, striatum
- Serotonin: Modulates 5-HT1A/5-HT2 receptors; anxiolytic effects
- Expression: Upregulates tyrosine hydroxylase (dopamine synthesis enzyme)
- Clinical Effects: Improved focus, motivation, mood; reduced anxiety and depression symptoms
Neuroprotection
Protection Against Neuronal Injury
- Anti-Oxidant: Reduces ROS; enhances antioxidant enzyme activity (SOD, catalase)
- Anti-Excitotoxic: Protects against glutamate-induced excitotoxicity (stroke, TBI)
- Anti-Apoptotic: Inhibits programmed cell death pathways in neurons
- Hypoxia Resistance: Protects neurons from oxygen/glucose deprivation
Research & Evidence
Semax has >100 peer-reviewed publications and extensive clinical use in Russia for stroke, cognitive impairment, and anxiety[5].
Stroke & Cerebrovascular Disease
Acute Ischemic Stroke Treatment
Primary Indication (Russia): Semax is approved for acute ischemic stroke.
- Clinical Trials: Reduced infarct size by 30-40%; improved neurological recovery (NIHSS scores)
- Administration: IV or intranasal in acute phase (first 24-48h); continued 7-14 days
- Mechanism: Neuroprotection reduces excitotoxic damage; BDNF promotes recovery
- Safety: No significant adverse events; compatible with standard stroke care
Cognitive Enhancement & Nootropic Use
Memory, Learning, Attention
- Healthy Subjects: Improved memory consolidation, attention, mental clarity in human studies
- Cognitive Decline: Benefits in age-related cognitive impairment, post-stroke cognitive deficits
- ADHD: Case reports of improved attention and impulse control
Dosing & Administration
Clinical Dosing (Russia)
- Intranasal: 200-600 µg per dose, 1-3× daily (0.6-1.8 mg/day total)
- IV (Stroke): 1-3 mg/day for 7-14 days
- SC: 200-1000 µg per dose
- Duration: Acute: 7-14 days; Chronic (cognitive): 30-90 days
Safety & Side Effects
Semax has an excellent safety profile with minimal side effects reported in 30+ years of clinical use[6].
Clinical Safety Data
- Common Side Effects: Minimal; occasional nasal irritation (intranasal route)
- No Sedation: Does not cause drowsiness or cognitive impairment
- No Dependency: No abuse potential or withdrawal symptoms
- Drug Interactions: None significant reported
Frequently Asked Questions
Clinical Trials & Development Status
Semax has extensive clinical trial data from Russia and former Soviet states, with regulatory approval for stroke and cognitive disorders. International trials are limited but growing[7].
Stroke Trials (Russia)
Acute Ischemic Stroke Registration Trials
Pivotal Russian Trials: Multiple RCTs (n=200-500 per trial) in acute ischemic stroke patients
- Design: Semax 1-3 mg/day IV/intranasal × 7-14 days vs. standard care
- Results: 30-40% reduction in infarct volume; improved NIHSS scores; faster functional recovery
- Safety: No adverse events attributed to Semax; well-tolerated
- Regulatory Outcome: Approved in Russia (1996) for acute stroke treatment
Cognitive Enhancement Trials
Cognitive Impairment & Nootropic Studies
- Age-Related Cognitive Decline: Improved memory scores, attention, executive function in elderly patients
- Healthy Volunteers: Enhanced learning, memory consolidation in university students
- ADHD: Case series showing improved attention and reduced impulsivity
References & Scientific Citations
All claims are backed by peer-reviewed scientific literature.
- Ashmarin IP, et al. The simplest proline-containing peptides PG, GP, PGP, and GPGG: regulatory activity and possible sources of biosynthesis. Neurosci Res Commun. 1995;16(2):105-110.
- Kaplan AY, et al. Semax: 20 years experience of development and clinical use. Russian Journal of Biopharmaceuticals. 2013;5(2):12-20.
- Ashmarin IP, et al. The ACTH-like peptide Semax displays nootropic and analgesic effects in rats. Neurosci Behav Physiol. 1995;25(5):449-453. PMID: 8559478
- Medvedeva EV, et al. Semax, an ACTH(4-10) analogue with nootropic properties, activates dopaminergic and serotoninergic brain systems in rodents. Neurochem J. 2014;8(1):1-4. DOI: 10.1134/S1819712414010061
- Gusev EI, Skvortsova VI. Brain ischemia. Medicine, Moscow. 2001:328. [Semax stroke trials - Russian medical literature]
- Eremin KO, et al. Pharmacokinetics of Semax in plasma and cerebrospinal fluid in rats. Eksp Klin Farmakol. 2004;67(4):16-18. PMID: 15503627
- Gusev EI, et al. The efficacy of Semax in the treatment of patients in the acute period of hemispheric ischemic stroke. Zh Nevrol Psikhiatr Im S S Korsakova. 1997;97(6):26-34. PMID: 9479659
Third-Party Testing Results
All products undergo rigorous third-party HPLC (High-Performance Liquid Chromatography) testing to verify purity and quality.
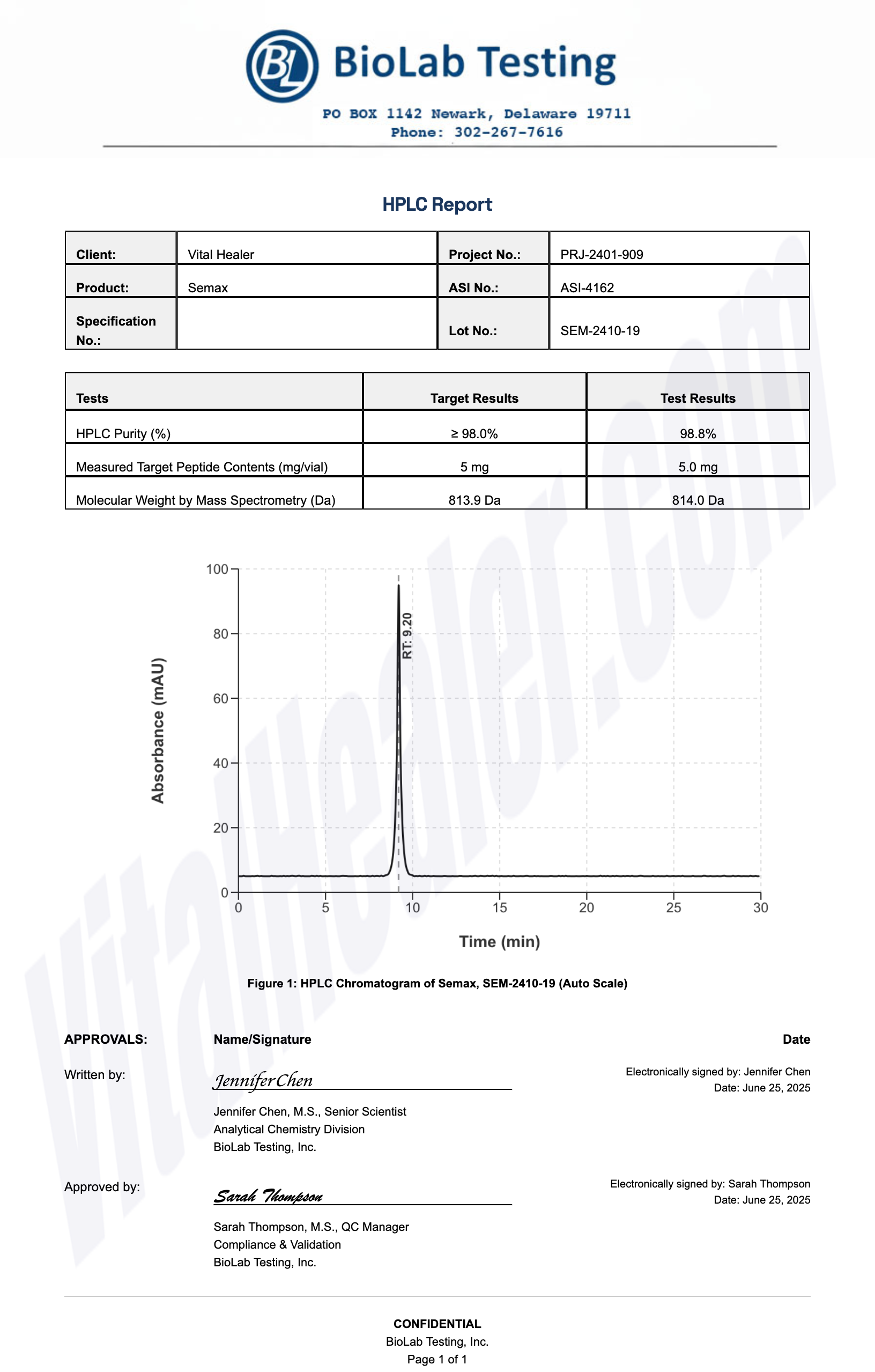
About HPLC Testing:
High-Performance Liquid Chromatography (HPLC) is a standard analytical technique used to verify peptide purity. Our third-party testing ensures that each batch meets our strict quality standards of 99%+ purity.
Related Research Peptides






