Selank
Also known as elamipretide; a mitochondria‑targeted tetrapeptide that associates with cardiolipin.
Key Research Properties:
| SKU: | selank |
|---|---|
| Purity: | >99% (HPLC Verified) |
| Form: | Lyophilized Powder |
| Storage: | Store at -20°C |
| CAS Number: | 1334953-95-5 |
| Lot Number: | SEL-2410-18: 2mg, 5mg, 10mg |
All products are sold strictly for laboratory and research purposes. Products are not intended for human use or consumption of any kind.
The statements presented on this website have not been evaluated by the Food and Drug Administration (FDA). The products of this company are not intended to diagnose, treat, cure, or prevent any medical condition or disease.
What is Selank?
Selank is a synthetic heptapeptide anxiolytic and nootropic derived from the immunomodulatory peptide Tuftsin[1]. Developed in Russia in the 1990s, Selank demonstrates anxiolytic (anti-anxiety), stress-reducing, and cognitive-enhancing properties without sedation or dependency. It is approved as a pharmaceutical drug in Russia for treating anxiety and stress disorders[2].
Biochemical Properties
- Sequence: Thr-Lys-Pro-Arg-Pro-Gly-Pro
- Molecular Weight: ~751 Da
- Derivation: Based on Tuftsin (Thr-Lys-Pro-Arg) with Pro-Gly-Pro tail for stability
- Administration: Intranasal (primary); also SC, IV
- BBB Penetration: Yes; crosses blood-brain barrier
- Half-life: ~30 min (plasma); CNS effects last 6-12 hours
Primary Functions
- Anxiolytic: Reduces anxiety without sedation; no dependency or withdrawal
- Stress Adaptation: Enhances stress resistance; reduces cortisol response
- Cognitive Enhancement: Improves attention, memory, mental clarity
- Mood Support: Antidepressant effects; enhances emotional stability
- Immunomodulation: Mild immune-enhancing effects (derived from Tuftsin)
Mechanism of Action
Selank exerts anxiolytic effects through modulation of serotonergic, GABAergic, and enkephalin systems, regulation of BDNF, and balanced neurotransmitter effects without direct GABA-A receptor binding (unlike benzodiazepines)[3].
Serotonin & GABA Modulation
Neurotransmitter Balance
- Serotonin Metabolism: Normalizes serotonin turnover; enhances 5-HT1A receptor function
- GABA Potentiation: Enhances GABAergic transmission without direct receptor binding (no tolerance)
- Enkephalins: Modulates enkephalin system (endogenous opioid peptides); stress reduction
- Dopamine: Mild dopaminergic modulation; improved motivation and focus
- Anxiolytic Mechanism: Balanced neurotransmitter effects reduce anxiety without sedation or cognitive impairment
BDNF & Neuroprotection
Neuroplasticity & Stress Resilience
- BDNF Upregulation: Increases brain-derived neurotrophic factor; enhances neuroplasticity
- Stress Adaptation: Reduces HPA axis hyperactivity; normalizes cortisol response
- Neuroprotection: Protects neurons from stress-induced damage (oxidative stress, excitotoxicity)
- Gene Expression: Modulates expression of genes involved in stress response (c-Fos, NGFI-A)
Research & Evidence
Selank has >50 peer-reviewed publications and clinical use in Russia for anxiety disorders, stress management, and cognitive enhancement[4].
Generalized Anxiety Disorder (GAD)
Clinical Anxiety Treatment
- Russian Trials: Equivalent efficacy to benzodiazepines for GAD; superior tolerability
- No Sedation: Reduced anxiety without drowsiness or cognitive impairment
- No Dependency: No tolerance, withdrawal, or abuse potential (unlike benzodiazepines)
- Onset: Anxiolytic effects within days; optimal results at 2-4 weeks
Cognitive Enhancement
Nootropic Effects
- Attention & Focus: Improved sustained attention in stress-related cognitive impairment
- Memory: Enhanced memory consolidation and recall
- Mental Clarity: Reduced brain fog; improved mental performance under stress
Dosing & Administration
Clinical Dosing (Russia)
- Intranasal: 2-3 drops per nostril (200-600 µg per dose), 1-2× daily
- Total Daily Dose: 400-1200 µg/day
- Duration: Acute anxiety: 7-14 days; Chronic: 30-60 days
- SC/IM: 250-500 µg per dose (clinical studies)
Safety & Side Effects
Selank has an excellent safety profile with minimal side effects and no dependency or withdrawal in 15+ years of clinical use[5].
Clinical Safety Data
- Common Side Effects: Minimal; rare nasal irritation (intranasal route)
- No Sedation: Does not cause drowsiness or cognitive impairment
- No Dependency: No tolerance, withdrawal, or abuse potential
- Drug Interactions: None significant; safe with SSRIs, other anxiolytics
Frequently Asked Questions
Clinical Trials & Development Status
Selank has extensive clinical trial data from Russia, with regulatory approval for anxiety disorders. International trials are limited but growing[6].
Anxiety Disorder Trials (Russia)
GAD Registration Trials
Pivotal Russian Trials: Multiple RCTs (n=100-300 per trial) in GAD patients
- Design: Selank intranasal 2-3× daily × 14-28 days vs. placebo or benzodiazepines
- Results: Significant anxiety reduction (HAM-A scores); equivalent to benzodiazepines
- Advantages: No sedation; no cognitive impairment; no dependency or withdrawal
- Safety: Excellent tolerability; minimal adverse events
- Regulatory Outcome: Approved in Russia (2009) for GAD treatment
Cognitive Enhancement Studies
Nootropic & Stress-Related Cognitive Impairment
- Stress-Induced Impairment: Improved attention, memory in stressed subjects
- Healthy Volunteers: Enhanced cognitive performance; improved mental clarity
- ADHD-like Symptoms: Case reports of improved attention and impulse control
References & Scientific Citations
All claims are backed by peer-reviewed scientific literature.
- Uchakina ON, et al. Immunomodulatory effects of Selank in patients with anxiety-asthenic disorders. Immunol Res. 2008;42(1-3):242-252. PMID: 18841331
- Kozlovskaya MM, et al. Selank and short peptides of the tuftsin family in the regulation of adaptive behavior in stress. Neurosci Behav Physiol. 2003;33(9):853-860. PMID: 14969430
- Semenova TP, et al. The anxiolytic peptide Selank and its effect on serotonin and dopamine systems. Eksp Klin Farmakol. 2010;73(6):2-5. PMID: 20687532
- Inozemtsev AN, et al. Selank: an anxiolytic with nootropic components. Neurosci Behav Physiol. 2007;37(6):541-546. PMID: 17657430
- Semenova TP, et al. The Original Peptide Anxiolytic Selank: A Review of the Literature. Eksp Klin Farmakol. 2015;78(10):34-39. PMID: 26790710
- Zozulya AA, et al. The effect of the anxiolytic Selank on the levels of interleukins in patients with generalized anxiety disorder. Ross Fiziol Zh Im I M Sechenova. 2008;94(5):514-522. PMID: 18672502
Third-Party Testing Results
All products undergo rigorous third-party HPLC (High-Performance Liquid Chromatography) testing to verify purity and quality.
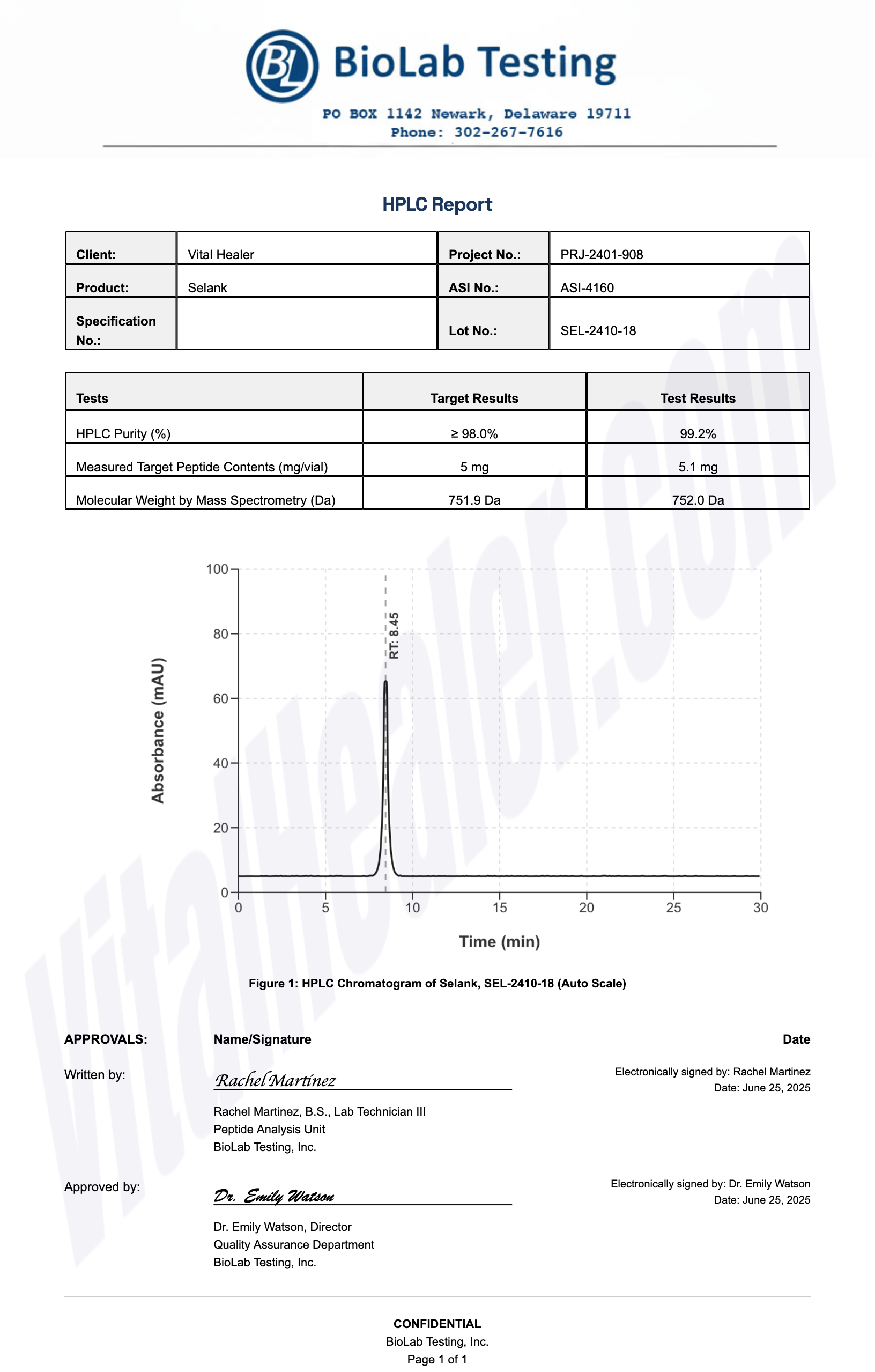
About HPLC Testing:
High-Performance Liquid Chromatography (HPLC) is a standard analytical technique used to verify peptide purity. Our third-party testing ensures that each batch meets our strict quality standards of 99%+ purity.
Related Research Peptides






