PT-141 (Bremelanotide)
A melanocortin MC4R receptor agonist derived from Melanotan II. Investigated for central nervous system modulation of sexual function. The FDA-approved formulation (Vyleesi®) treats hypoactive sexual desire disorder in premenopausal women.
Key Research Properties:
| SKU: | pt-141-bremelanotide |
|---|---|
| Purity: | >99% (HPLC Verified) |
| Form: | Lyophilized Powder |
| Storage: | Store at -20°C |
| CAS Number: | 189691-06-3 |
| Lot Number: | PT1-2410-16: 10mg |
All products are sold strictly for laboratory and research purposes. Products are not intended for human use or consumption of any kind.
The statements presented on this website have not been evaluated by the Food and Drug Administration (FDA). The products of this company are not intended to diagnose, treat, cure, or prevent any medical condition or disease.
Overview
PT-141 (Bremelanotide) is a cyclic heptapeptide melanocortin receptor agonist that acts centrally through the hypothalamus to modulate sexual arousal and desire pathways. Originally derived from the α-melanocyte-stimulating hormone (α-MSH) analog Melanotan II, bremelanotide was specifically developed to target sexual function without the tanning effects associated with its predecessor.[1]
Chemical Structure and Properties
PT-141 is characterized by its cyclic peptide structure, distinguishing it from linear peptides and conferring unique pharmacological properties:
Molecular Specifications
- Molecular Formula: C₅₀H₆₈N₁₄O₁₀
- Molecular Weight: 1025.2 g/mol
- CAS Number: 189691-06-3
- Sequence: Ac-Nle-cyclo[Asp-His-D-Phe-Arg-Trp-Lys]-OH
- Structure: Cyclic heptapeptide
Key Characteristics
- Receptor Affinity: Non-selective melanocortin agonist (MC1R, MC3R, MC4R, MC5R)
- Route: Subcutaneous injection
- Half-life: ~2.7 hours
- Onset: Effects typically within 1-2 hours
- Duration: Up to 12-24 hours
Development History
The journey of PT-141 from research compound to FDA-approved medication spans over two decades of clinical investigation:
Discovery Phase
Bremelanotide was initially developed from Melanotan II at the University of Arizona, where researchers identified that this analog could induce sexual arousal effects independently of skin tanning. The compound was originally investigated for male erectile dysfunction but was redirected to female sexual dysfunction after early trial observations.[3]
Early Clinical Development
Initial Phase I and II trials were conducted using intranasal administration, evaluating efficacy in both male erectile dysfunction and female sexual arousal disorder. However, intranasal delivery was associated with significant blood pressure increases, leading to a clinical hold by the FDA and subsequent reformulation.[4]
Subcutaneous Formulation Development
Development shifted to a subcutaneous autoinjector formulation to avoid cardiovascular side effects. The pivotal Phase 3 program, known as RECONNECT, enrolled nearly 1,300 premenopausal women with HSDD across two identical, randomized, placebo-controlled trials (RECONNECT-1 and RECONNECT-2).[5]
FDA Approval
The FDA approved bremelanotide (Vyleesi®) as the first and only melanocortin receptor agonist for the treatment of acquired, generalized HSDD in premenopausal women. This marked a significant milestone as only the second FDA-approved medication specifically for female sexual dysfunction.[2]
Expanded Research & Clinical Use
Post-approval studies continue to evaluate bremelanotide's efficacy in broader populations, including postmenopausal women and men with erectile dysfunction. Additional research explores combination therapies with PDE5 inhibitors and potential applications beyond sexual dysfunction.[6]
Primary Research Applications
The FDA-approved indication focuses on acquired, generalized HSDD in premenopausal women. Clinical trials demonstrated significant improvements in sexual desire, arousal, and distress scores compared to placebo.[5]
Research investigates bremelanotide for erectile dysfunction, particularly in men who do not respond to PDE5 inhibitors. Studies explore monotherapy and combination approaches with existing ED treatments.[7]
As a centrally-acting agent, bremelanotide provides insights into hypothalamic regulation of sexual behavior, offering a distinct mechanism from peripherally-acting medications.[8]
Mechanism of Action
PT-141 (Bremelanotide) functions as a non-selective melanocortin receptor agonist, acting primarily through central nervous system pathways to modulate sexual behavior. Unlike peripherally-acting agents that enhance physical aspects of sexual function (such as blood flow), bremelanotide targets the neurological basis of sexual desire and arousal in the hypothalamus and limbic system.[8]
Melanocortin Receptor System
The melanocortin receptor family consists of five G-protein coupled receptors (MC1R-MC5R), each with distinct tissue distribution and physiological roles. Bremelanotide exhibits agonist activity at multiple melanocortin receptors, though its sexual function effects are primarily attributed to MC3R and MC4R activation:
| Receptor | Primary Location | Key Functions | Bremelanotide Activity |
|---|---|---|---|
| MC1R | Melanocytes, immune cells | Pigmentation, inflammation | Agonist |
| MC2R | Adrenal cortex | Steroidogenesis (ACTH receptor) | Minimal |
| MC3R | Hypothalamus, limbic system | Energy homeostasis, sexual behavior | Primary Target |
| MC4R | CNS (widespread) | Appetite, sexual function, autonomic regulation | Primary Target |
| MC5R | Peripheral tissues, sebaceous glands | Exocrine gland function | Agonist |
Central Nervous System Effects
Hypothalamic Regulation
Bremelanotide activates MC3R and MC4R in the hypothalamus, particularly in the paraventricular nucleus (PVN). This activation initiates a cascade of neuronal signaling that modulates sexual motivation and arousal independent of peripheral genital stimulation.[9]
Key Effects:
- Increases dopaminergic tone in reward pathways
- Modulates oxytocin and vasopressin release
- Enhances neural signaling to spinal erectile centers
Limbic System Activation
Beyond the hypothalamus, bremelanotide influences limbic structures involved in emotional processing, motivation, and sexual arousal. These areas include the amygdala, nucleus accumbens, and ventral tegmental area (VTA).[10]
Key Effects:
- Enhances sexual motivation and incentive salience
- Modulates emotional valence of sexual stimuli
- Increases attention to sexual cues
Cellular Signaling Pathways
At the cellular level, bremelanotide binding to melanocortin receptors initiates G-protein coupled receptor (GPCR) signaling cascades:
Signal Transduction Cascade
- Receptor Binding: Bremelanotide binds to MC3R/MC4R on the cell surface
- G-Protein Activation: Receptor conformational change activates Gs proteins
- cAMP Production: Activated Gs stimulates adenylyl cyclase, increasing intracellular cyclic AMP (cAMP)
- PKA Activation: Elevated cAMP activates protein kinase A (PKA)
- CREB Phosphorylation: PKA phosphorylates CREB (cAMP response element-binding protein)
- Gene Transcription: Phosphorylated CREB promotes transcription of genes involved in neuronal excitability and neurotransmitter synthesis
- Physiological Response: Changes in neuronal firing patterns, neurotransmitter release, and downstream sexual behavior pathways
Pharmacokinetics & Pharmacodynamics
Absorption & Distribution
- Route: Subcutaneous injection
- Bioavailability: ~100% (subcutaneous)
- Tmax: Approximately 1 hour post-injection
- Distribution: Crosses blood-brain barrier to reach CNS targets
- Protein Binding: Minimal (peptide structure)
Metabolism & Elimination
- Metabolism: Proteolytic degradation (non-CYP450)
- Half-life: Approximately 2.7 hours
- Duration of Action: Effects may persist 12-24 hours despite short half-life
- Excretion: Renal elimination of metabolites
- Drug Interactions: Minimal due to non-hepatic metabolism
Distinction from Other Treatments
Bremelanotide's central mechanism distinguishes it from other sexual dysfunction therapies:[11]
| Treatment Class | Example | Mechanism | Primary Target |
|---|---|---|---|
| Melanocortin Agonist | Bremelanotide (PT-141) | MC3R/MC4R activation in CNS | Sexual desire & arousal (central) |
| PDE5 Inhibitors | Sildenafil, Tadalafil | cGMP degradation inhibition | Erectile function (peripheral) |
| Dopamine Agonist | Flibanserin | 5-HT1A agonist, 5-HT2A antagonist | Sexual desire (central) |
| Hormonal | Testosterone | Androgen receptor activation | Multiple (systemic) |
Research Applications
PT-141 (Bremelanotide) has been extensively investigated across multiple clinical indications, with the most robust evidence supporting its use in female sexual dysfunction. Research continues to explore its potential in male sexual dysfunction, combination therapies, and other melanocortin-mediated conditions.
Female Sexual Dysfunction
The pivotal RECONNECT clinical trial program established bremelanotide's efficacy for hypoactive sexual desire disorder (HSDD) in premenopausal women, leading to FDA approval in 2019.[5]
RECONNECT-1 and RECONNECT-2 Trials
Study Design: Two identical, multicenter, randomized, double-blind, placebo-controlled Phase 3 trials
Population: 1,247 premenopausal women (aged 18-50) with acquired, generalized HSDD
Intervention: Subcutaneous bremelanotide 1.75 mg vs. placebo, administered on-demand before anticipated sexual activity (maximum 1 dose per 24 hours, 8 doses per month)
Duration: 24 weeks
Primary Endpoints & Results:
- Sexual Desire: Significant improvement in the Female Sexual Function Index (FSFI) Desire domain score compared to placebo (p < 0.001 in both trials)
- Distress: Significant reduction in the Female Sexual Distress Scale-Desire/Arousal/Orgasm (FSDS-DAO) Item 13 (distress related to low sexual desire) compared to placebo (p < 0.001 in both trials)
Secondary Endpoints:
- Increased number of satisfying sexual events
- Improved overall sexual function scores
- Enhanced arousal, lubrication, and satisfaction domains
- Reduced overall sexual distress
Clinical Significance: The RECONNECT trials demonstrated that bremelanotide addresses both components of HSDD—low sexual desire and associated distress—making it the first centrally-acting, on-demand treatment for this condition.[5]
Postmenopausal Women Research
While FDA approval is currently limited to premenopausal women, research has also evaluated bremelanotide in postmenopausal populations:
- Phase 2 studies in postmenopausal women with HSDD showed improvements in sexual desire and arousal similar to those observed in premenopausal cohorts[12]
- Efficacy appeared consistent regardless of menopausal status, suggesting potential broader applications
- Further Phase 3 trials in postmenopausal women may expand the approved indication
Male Erectile Dysfunction
Although bremelanotide's development initially targeted male erectile dysfunction, cardiovascular concerns with intranasal administration led to a shift in focus. Recent studies have revisited its potential in men using the safer subcutaneous route:[7]
Monotherapy Studies
Population: Men with erectile dysfunction (organic, psychogenic, or mixed etiology)
Findings:
- Moderate improvements in erectile function scores
- Benefits most pronounced in men with psychogenic ED
- Central arousal enhancement may complement peripheral mechanisms
Limitation: Efficacy generally lower than PDE5 inhibitors for purely organic ED
Combination with PDE5 Inhibitors
Population: Men with ED who are partial or non-responders to PDE5 inhibitors alone
Findings:
- Enhanced erectile response when combined with sildenafil or tadalafil[13]
- Synergistic effects: central arousal (bremelanotide) + peripheral vasodilation (PDE5i)
- Improved outcomes in difficult-to-treat ED populations
Status: Ongoing Phase 2 trials evaluating optimal combination dosing regimens
Neurobiological Research
Beyond clinical efficacy trials, bremelanotide serves as a valuable research tool for understanding the neurobiology of sexual behavior and desire:
| Research Area | Key Findings | Reference |
|---|---|---|
| fMRI Studies | Bremelanotide administration increases activation in hypothalamus, amygdala, and nucleus accumbens during sexual stimulus presentation | [14] |
| Neurotransmitter Modulation | MC4R activation enhances dopamine release in mesolimbic reward pathways; modulates oxytocin and vasopressin systems | [9] |
| Sexual Dimorphism | Sex differences in melanocortin receptor expression and downstream signaling may explain differential responses between men and women | [15] |
| Animal Models | Rodent studies confirm MC4R knockout abolishes bremelanotide's pro-sexual effects, validating receptor-specific mechanism | [16] |
Emerging Research Directions
While erectile dysfunction receives significant attention, low sexual desire in men is increasingly recognized as a distinct clinical entity. Early-phase studies suggest bremelanotide may address desire deficits in men, particularly those with normal erectile function but reduced libido.[17]
Transgender individuals undergoing hormone therapy often experience sexual dysfunction. Preliminary research explores whether bremelanotide's central mechanism could address desire and arousal concerns in gender-affirming hormone therapy patients.[18]
Antidepressants (particularly SSRIs) and antihypertensives frequently cause sexual side effects. Research investigates whether bremelanotide can counteract medication-induced sexual dysfunction by bypassing the mechanisms affected by these drugs.[19]
Clinical trials are evaluating bremelanotide's efficacy when integrated with behavioral interventions and couples counseling. The hypothesis is that pharmacological enhancement of desire combined with therapeutic techniques may yield superior outcomes to either approach alone.[20]
Special Populations
Cancer Survivors
Sexual dysfunction is common after cancer treatment. Pilot studies explore bremelanotide for HSDD in breast cancer survivors and men post-prostatectomy.[21]
Spinal Cord Injury
Central mechanism may benefit individuals with SCI who experience neurological barriers to sexual function. Early-phase research is ongoing.[22]
Diabetes & Metabolic Syndrome
Sexual dysfunction is prevalent in diabetes. Research evaluates whether bremelanotide's CNS action can overcome peripheral vascular and neurological complications.[23]
Dosing Information
Bremelanotide was approved by the FDA in June 2019 under the brand name Vyleesi® for the treatment of hypoactive sexual desire disorder (HSDD) in premenopausal women. The approved formulation is a subcutaneous autoinjector for on-demand use prior to anticipated sexual activity.[24]
FDA-Approved Dosing (Vyleesi®)
Standard Dosing Protocol
Dose: 1.75 mg subcutaneous injection
Route: Subcutaneous (abdomen or thigh)
Timing: At least 45 minutes before anticipated sexual activity
Frequency: Maximum 1 dose per 24 hours
Maximum Monthly: 8 doses per month
Onset: Effects typically begin within 30-60 minutes
Duration: Peak effect 1-3 hours; may persist 6-8 hours
Device: Single-dose, prefilled autoinjector
Storage: Refrigerate (2-8°C); may be kept at room temperature up to 77°F for up to 7 days
Administration Technique
Injection Sites
Preferred Sites:
- Abdomen: At least 2 inches away from navel, avoiding areas with scars or stretch marks
- Thigh: Front or outer portion, mid-thigh region
Technique:
- Rotate injection sites to minimize irritation
- Pinch skin and insert needle at 90-degree angle
- Hold for 5 seconds after injection
- Do not inject into areas with active skin conditions
Patient Instructions
Before Administration:
- Remove from refrigerator 15-30 minutes prior to allow warming to room temperature
- Inspect solution for discoloration or particles (should be clear, colorless to pale yellow)
- Wash hands and clean injection site with alcohol swab
After Administration:
- Dispose of autoinjector in sharps container
- Monitor for injection site reactions
- Note timing and response for future reference
Clinical Trial Dosing Protocols
Clinical development of bremelanotide explored various doses and routes before settling on the FDA-approved regimen:
| Study Phase | Route | Dose Range | Key Findings | Status |
|---|---|---|---|---|
| Early Phase 2 | Intranasal | 10-20 mg | Efficacy demonstrated but cardiovascular side effects (transient hypertension) led to discontinuation of this route | Discontinued |
| Phase 2 (SC) | Subcutaneous | 0.75-2.0 mg | Dose-response relationship established; 1.75 mg identified as optimal balance of efficacy and tolerability | Completed |
| RECONNECT Phase 3 | Subcutaneous | 1.75 mg as needed | Pivotal trials demonstrating efficacy and safety; led to FDA approval | Approved |
| Male ED Studies | Subcutaneous | 1.75-2.5 mg | Moderate efficacy in psychogenic ED; ongoing Phase 2 combination studies | Phase II |
Timing & Response Optimization
Clinical trials established that administration at least 45 minutes before anticipated sexual activity is optimal. However, individual responses vary:
- Early Responders (30-45 min): ~30% of women report onset of effect within this window
- Typical Response (45-90 min): Majority experience peak effects during this period
- Delayed Response (>90 min): Some individuals require longer onset; effects may persist for several hours
Recommendation: Patients should experiment with timing during initial doses to identify their personal response pattern.[26]
Maximum Frequency: FDA approval limits use to 1 dose per 24 hours, with a maximum of 8 doses per month. This restriction is based on:
- Safety data from clinical trials (no long-term daily dosing studies)
- Pharmacodynamic concerns (potential receptor desensitization with frequent use)
- Side effect profile (nausea, flushing more common with frequent dosing)
Tachyphylaxis: No evidence of tolerance (reduced efficacy) with chronic on-demand use at recommended frequencies. Long-term extension studies showed sustained efficacy over 12 months.[27]
Food: Bremelanotide can be administered with or without food. Subcutaneous absorption is not significantly affected by meal timing.
Alcohol:
- No pharmacokinetic interactions with moderate alcohol consumption
- However, alcohol may exacerbate side effects (nausea, flushing, dizziness)
- Excessive alcohol impairs sexual function independently and may reduce bremelanotide efficacy
Recommendation: Limit alcohol to moderate consumption when using bremelanotide.
Special Populations & Dose Adjustments
Renal Impairment
Mild-Moderate (CrCl ≥30 mL/min): No dose adjustment required. Bremelanotide is predominantly metabolized; renal clearance plays minor role.
Severe (CrCl <30 mL/min): Not studied. Use with caution; consider avoiding due to lack of safety data.
Dialysis: No data available; bremelanotide unlikely to be dialyzable due to peptide structure and volume of distribution.
Hepatic Impairment
Mild-Moderate (Child-Pugh A-B): No dose adjustment required. Pharmacokinetic studies showed minimal impact of hepatic dysfunction on bremelanotide exposure.
Severe (Child-Pugh C): Not studied. Use with caution and consider avoiding in decompensated cirrhosis.
Mechanism: Bremelanotide undergoes proteolytic degradation rather than hepatic metabolism, minimizing liver's role in clearance.
Geriatric Population (≥65 years)
No specific dose adjustments recommended based on age. However:
- Limited clinical trial data in women >65 years
- Age-related physiological changes may affect response
- Higher prevalence of cardiovascular risk factors requires careful assessment
Recommendation: Standard dosing with careful monitoring for side effects, particularly cardiovascular symptoms.
Body Weight Considerations
Obesity: No dose adjustment required. Pharmacokinetic analyses showed no clinically significant impact of BMI on bremelanotide exposure.
Low Body Weight (<50 kg): Limited data; standard dose generally well-tolerated but monitor for increased side effect incidence.
Clinical Note: RECONNECT trials included women across wide BMI range (18-40 kg/m²) with consistent efficacy.[28]
Missed Dose & Discontinuation
Dosing Guidance
Missed Dose: Not applicable—bremelanotide is used on-demand, not on a fixed schedule. Patients simply administer when planning sexual activity.
Overdose:
- No cases of serious overdose reported in clinical trials
- Expected effects of excessive dosing: exaggerated pharmacological effects (nausea, vomiting, flushing, hypotension)
- Treatment: supportive care, monitoring of vital signs, hydration
- No specific antidote available
Discontinuation: No withdrawal symptoms or rebound effects observed upon discontinuation. Patients can stop at any time without tapering.
Safety Profile
Bremelanotide has been extensively evaluated in clinical trials involving over 1,200 women in the pivotal RECONNECT studies, plus thousands more in earlier-phase and post-marketing surveillance. The safety profile is generally favorable, with most adverse events being mild to moderate and transient.[29]
Clinical Trial Safety Data
| Safety Parameter | RECONNECT Trials | Long-Term Extension |
|---|---|---|
| Total Exposure | ~1,200 women (24 weeks) | ~600 women (52 weeks) |
| Treatment Discontinuation (AEs) | 13% (bremelanotide) vs. 2% (placebo) | 18% over 52 weeks |
| Serious Adverse Events | <1% (similar to placebo) | <2% |
| Deaths | None attributed to bremelanotide | None |
| Cardiovascular Events | No increase vs. placebo (SC route) | No signals detected |
Common Adverse Events
The following adverse events occurred in ≥2% of patients treated with bremelanotide and more frequently than placebo in the RECONNECT trials:
Very Common (>10%)
- Nausea: 40% (most common; usually mild-moderate, transient, decreases with repeat dosing)[30]
- Flushing: 20% (facial warmth, redness; typically resolves within 1-2 hours)
- Injection Site Reactions: 13% (erythema, pain, bruising at injection site; usually mild)
- Headache: 11% (tension-type or migraine-like; often co-occurs with nausea)
Common (2-10%)
- Vomiting: 5% (usually accompanies severe nausea; rare after first few doses)
- Hot Flush: 4% (distinct from facial flushing; whole-body warmth sensation)
- Nasal Congestion: 3% (despite SC route; likely melanocortin effect on nasal vasculature)
- Toothache: 2% (unusual adverse event, mechanism unclear)
- Dizziness: 2% (postural or non-specific; resolve spontaneously)
Cardiovascular Safety
Blood Pressure & Heart Rate Effects
Historical Context: Early development of bremelanotide used intranasal administration, which was associated with transient blood pressure elevations (mean increase ~10-15 mmHg systolic). This led to discontinuation of the intranasal formulation and shift to subcutaneous route.[31]
Subcutaneous Route Safety:
- Blood Pressure: Transient, mild increases in BP observed in some patients (mean increase <5 mmHg systolic); clinically significant hypertension rare (<1%)
- Heart Rate: Small increases in HR (mean increase 4-6 bpm); no clinically significant tachycardia
- Duration: Cardiovascular effects peak 1-2 hours post-dose and return to baseline within 12 hours
- High-Risk Patients: Patients with uncontrolled hypertension or significant cardiovascular disease were excluded from trials; use with caution in these populations
FDA Recommendation: Temporary increase in blood pressure or decrease in heart rate may occur with each dose. Advise patients to seek medical attention if symptoms of severe hypertension occur (chest pain, shortness of breath, severe headache).
Contraindications & Warnings
- Uncontrolled Hypertension: Blood pressure >140/90 mmHg; risk of exacerbating hypertensive episodes
- Known Cardiovascular Disease: MI, stroke, or life-threatening arrhythmia within past 6 months
- Pregnancy: Not indicated in pregnancy; animal studies show potential fetal harm; women of childbearing potential should use contraception
- Postmenopausal Women: Not approved for this population (FDA approval limited to premenopausal women)
Darkening of Skin: Melanocortin receptor activation can increase melanin production. Focal hyperpigmentation (face, gingiva, breasts) reported in <1% of patients. Generally reversible upon discontinuation but may persist.
Nausea: Can be severe; antiemetics (e.g., ondansetron) may be considered for prophylaxis if nausea limits use.
Syncope/Presyncope: Rare reports (<1%); likely related to vasodilation. Advise patients to sit or lie down if feeling lightheaded.
Lactation: Unknown if excreted in breast milk; peptide structure suggests low oral bioavailability to infant, but data lacking. Consider risk-benefit.
Although bremelanotide is not FDA-approved for men, ongoing research in male ED warrants consideration of cardiovascular safety:
- Intranasal formulation was discontinued in men due to BP concerns
- SC route shows better CV safety profile but still requires caution in high-risk men
- Men with recent MI, unstable angina, or uncontrolled hypertension should not use bremelanotide
- Sexual activity itself poses CV risk; assess baseline fitness for sexual activity
Drug Interactions
Low Interaction Potential
Bremelanotide is a peptide that undergoes proteolytic degradation and does not interact with cytochrome P450 enzymes. This minimizes potential for pharmacokinetic drug-drug interactions.
Studied Interactions (No Clinically Significant Effects):
- Oral Contraceptives: No effect on ethinyl estradiol or levonorgestrel exposure
- Alcohol: No pharmacokinetic interaction, though may worsen nausea/dizziness
- Other Sexual Dysfunction Drugs: No studies combining with flibanserin or PDE5 inhibitors; theoretical concern for additive hypotensive effects
Theoretical Interactions (Caution Advised):
- Antihypertensives: Additive blood pressure lowering possible; monitor BP
- Naltrexone: Opioid antagonists may theoretically modulate melanocortin signaling; clinical significance unknown
Pregnancy & Lactation
Pregnancy
Pregnancy Category: Not categorized under new FDA system; labeled with specific risk information
Animal Data: Adverse developmental outcomes observed in rats and rabbits at doses producing systemic exposures similar to human therapeutic dose
Human Data: No adequate studies in pregnant women
Recommendation: Contraindicated in pregnancy. Women of childbearing potential should use effective contraception. Discontinue immediately if pregnancy occurs.
Lactation
Excretion in Milk: Unknown (no human data)
Theoretical Considerations:
- Molecular weight (1025 Da) and peptide structure suggest limited passage into milk
- If present in milk, oral bioavailability to infant likely very low due to GI proteolysis
Recommendation: Consider risks vs. benefits. If bremelanotide is deemed necessary, consider pumping and discarding milk for 12 hours post-dose.
Risk-Benefit Analysis
Benefits (Evidence-Based)
- Significant improvements in sexual desire and associated distress (RECONNECT trials)
- On-demand dosing allows flexibility and control
- Central mechanism addresses desire deficit (not just physical arousal)
- Generally well-tolerated with manageable side effects
- No significant drug-drug interactions
- Effects typically diminish with repeated use
Risks & Considerations
- High incidence of nausea (40%) may limit tolerability
- Requires subcutaneous injection (barrier for needle-averse patients)
- Cardiovascular effects require caution in at-risk populations
- Not approved for postmenopausal women or men
- Long-term safety beyond 52 weeks not extensively studied
- Cost considerations (branded medication)
Frequently Asked Questions
PT-141 (bremelanotide) is a melanocortin receptor agonist that works through the central nervous system to enhance sexual desire and arousal. Unlike peripherally-acting medications (such as sildenafil/Viagra for men, which increases blood flow), bremelanotide acts in the brain's hypothalamus and limbic system to modulate neural pathways involved in sexual motivation.
Key Differences:
- vs. PDE5 Inhibitors (Viagra, Cialis): These work peripherally to enhance blood flow; bremelanotide works centrally on desire/arousal pathways
- vs. Flibanserin (Addyi): Flibanserin is taken daily and modulates serotonin/dopamine; bremelanotide is on-demand and acts via melanocortin receptors
- vs. Hormone Therapy: HT addresses hormonal deficiencies; bremelanotide enhances desire regardless of hormone levels
Onset: Effects typically begin within 30-60 minutes after subcutaneous injection, though individual responses vary. The FDA recommends administering at least 45 minutes before anticipated sexual activity.
Peak Effect: Maximum effects usually occur 1-3 hours after administration.
Duration: Effects may persist for 6-8 hours, though this varies significantly between individuals.
Important Note: Unlike PDE5 inhibitors that require sexual stimulation to work, bremelanotide enhances spontaneous desire and arousal, meaning effects occur regardless of external stimulation (though context and partner interaction still matter).
Most Common Side Effects:
- Nausea (40%): Usually mild-moderate, peaks 1-2 hours post-dose, resolves within 4-6 hours
- Flushing (20%): Facial warmth/redness, typically lasts 1-2 hours
- Injection site reactions (13%): Mild pain, redness, or bruising
- Headache (11%): Often co-occurs with nausea
Minimization Strategies:
- Side effects typically decrease significantly after the first 2-3 doses
- Consider anti-nausea medication (ondansetron) for first few doses
- Stay well-hydrated before and after administration
- Avoid alcohol, which may worsen nausea and dizziness
- Rotate injection sites to minimize local reactions
Bremelanotide is not currently FDA-approved for men. The FDA approval is specifically for hypoactive sexual desire disorder (HSDD) in premenopausal women only.
However, research in men is ongoing:
- Early studies showed bremelanotide could improve erectile function, particularly in psychogenic ED
- Development for male ED was paused due to cardiovascular concerns with the intranasal formulation
- Recent Phase 2 studies using subcutaneous bremelanotide show moderate efficacy, especially when combined with PDE5 inhibitors
- Potential niche: men with low desire (hypoactive sexual desire) or PDE5 inhibitor non-responders
Current Status: Off-label use occurs but lacks regulatory approval. Clinical trials continue to evaluate safety and efficacy in male populations.
Bremelanotide is contraindicated in women with uncontrolled hypertension (BP >140/90 mmHg) and should be used with caution in those with cardiovascular disease.
Cardiovascular Effects:
- Subcutaneous bremelanotide causes small, transient increases in blood pressure (mean <5 mmHg systolic) that peak 1-2 hours post-dose
- The early intranasal formulation caused more significant BP elevations and was discontinued
- Heart rate may increase slightly (mean 4-6 bpm)
- Effects return to baseline within 12 hours
Who Should Avoid Bremelanotide:
- Uncontrolled or poorly controlled hypertension
- Recent myocardial infarction or stroke (within 6 months)
- Unstable angina or severe cardiac arrhythmias
Who May Use with Monitoring: Women with well-controlled hypertension or mild cardiovascular risk factors can typically use bremelanotide under medical supervision with periodic BP monitoring.
In the pivotal RECONNECT-1 and RECONNECT-2 trials involving over 1,200 premenopausal women with HSDD:
Primary Efficacy Endpoints (Change from Baseline):
- Sexual Desire (FSFI-desire domain): Bremelanotide +0.3 to +0.4 vs. Placebo +0.2 to +0.3 (statistically significant difference)
- Distress (FSDS-DAO): Bremelanotide -0.3 to -0.4 vs. Placebo -0.2 to -0.3 (statistically significant difference)
Response Rates:
- ~40-45% of women treated with bremelanotide reported meaningful improvement in desire
- ~35-40% of placebo-treated women also reported improvement (significant placebo effect in sexual dysfunction trials)
- Net benefit over placebo: ~8-10% absolute increase in response rate
Clinical Interpretation: While statistical significance was achieved, the absolute magnitude of benefit is modest. Individual responses vary widely—some women experience dramatic improvements, while others see minimal effect. The significant placebo response highlights the importance of psychological factors in sexual function.
Oral Contraceptives: Yes. Drug interaction studies showed that bremelanotide does not affect the pharmacokinetics of ethinyl estradiol or levonorgestrel (common contraceptive components). No dose adjustment of either medication is needed.
Hormone Replacement Therapy (HRT): While not specifically studied, no pharmacokinetic interactions are expected based on bremelanotide's metabolism (peptide degradation, not hepatic). Women on HRT can likely use bremelanotide safely.
Important Notes:
- Some hormonal contraceptives (particularly progestin-only pills) may contribute to low libido; discuss with your provider
- Bremelanotide is not contraindicated in postmenopausal women, but it is not FDA-approved for this population
- Women of childbearing potential should use effective contraception, as bremelanotide is contraindicated in pregnancy
Risk: Melanocortin receptors regulate melanin production in the skin. Focal hyperpigmentation (darkening) has been reported in <1% of patients in clinical trials, typically affecting the face, gingiva (gums), or breasts.
Mechanism: MC1R activation increases melanogenesis. However, bremelanotide primarily targets MC3R and MC4R (not MC1R), so pigmentation effects are generally minimal.
Key Points:
- Incidence is very low (<1%) with therapeutic dosing
- Hyperpigmentation is usually reversible upon discontinuation
- Patients with darker skin tones may be less likely to notice changes
- This is distinct from Melanotan II (MT-2), a non-selective melanocortin agonist designed to promote tanning
Recommendation: Monitor for any changes in skin pigmentation. If focal darkening occurs, discuss with your healthcare provider; discontinuation typically results in gradual fading over weeks to months.
Storage Requirements:
- Refrigeration: Store at 2-8°C (36-46°F) in original packaging
- Room Temperature: May be kept at up to 25°C (77°F) for up to 7 days if needed for travel
- Light Protection: Keep in the original box to protect from light
- Freezing: Do not freeze; discard if frozen
Shelf Life: Check expiration date on packaging. Typically 24 months from manufacture date when stored properly.
Before Use:
- Remove from refrigerator 15-30 minutes before use to allow warming to room temperature (more comfortable injection)
- Inspect solution—should be clear, colorless to pale yellow, with no particulates
- Discard if discolored, cloudy, or contains particles
Pharmacokinetic Interactions: There is no direct pharmacokinetic interaction between bremelanotide and alcohol. Alcohol does not significantly affect bremelanotide absorption, distribution, or elimination.
Pharmacodynamic Concerns:
- Exacerbation of Side Effects: Alcohol may worsen nausea, dizziness, and flushing associated with bremelanotide
- Hypotension Risk: Both alcohol and bremelanotide can cause vasodilation and blood pressure changes; combination may increase risk of lightheadedness or syncope
- Sexual Function: Excessive alcohol independently impairs sexual function and may reduce bremelanotide efficacy
Recommendations:
- Moderate alcohol consumption (1-2 drinks) is generally acceptable
- Avoid heavy alcohol intake when using bremelanotide
- If you experience significant nausea with bremelanotide, consider avoiding alcohol entirely on dosing days
Clinical Trials
Bremelanotide has been evaluated in an extensive clinical development program spanning over two decades, from early Phase 1 safety studies through pivotal Phase 3 efficacy trials that led to FDA approval in 2019. This tab summarizes key clinical trials and provides resources for finding additional studies.
Pivotal Phase 3 Trials (RECONNECT Program)
The RECONNECT clinical trial program formed the basis for FDA approval of bremelanotide (Vyleesi®) for treatment of hypoactive sexual desire disorder (HSDD) in premenopausal women.
RECONNECT-1 (NCT02333071)
Study Title: "Study of Bremelanotide to Treat Hypoactive Sexual Desire Disorder in Premenopausal Women"
Status: Completed
Phase: Phase 3
Enrollment: 1,267 women
Study Period: 2014-2016
Location: United States (multi-center)
Design: Randomized, double-blind, placebo-controlled
Duration: 24 weeks
Intervention: Bremelanotide 1.75 mg SC on-demand vs. placebo
Primary Endpoint: Change in sexual desire (FSFI-desire) and distress (FSDS-DAO)
Key Results:
- Sexual Desire: Statistically significant improvement in FSFI-desire domain score compared to placebo (p<0.001)
- Distress Reduction: Significant decrease in distress related to low sexual desire (FSDS-DAO score, p<0.001)
- Satisfying Sexual Events: Increase in frequency of satisfying sexual encounters
- Safety: Most common adverse event was nausea (40%), generally mild-moderate and transient
- Tolerability: 13% discontinued due to adverse events vs. 2% with placebo
Publication: Kingsberg SA, et al. J Womens Health. 2019;28(11):1483-1493.
RECONNECT-2 (NCT02338960)
Study Title: "A Second Study of Bremelanotide to Treat Hypoactive Sexual Desire Disorder in Premenopausal Women"
Status: Completed
Phase: Phase 3
Enrollment: 1,247 women
Study Period: 2015-2017
Location: United States (multi-center)
Design: Randomized, double-blind, placebo-controlled
Duration: 24 weeks
Intervention: Bremelanotide 1.75 mg SC on-demand vs. placebo
Primary Endpoint: Change in sexual desire (FSFI-desire) and distress (FSDS-DAO)
Key Results:
- Replication of RECONNECT-1: Confirmed statistically significant improvements in both primary endpoints
- Consistency: Results nearly identical to RECONNECT-1, demonstrating reproducibility of effect
- Subgroup Analyses: Efficacy consistent across age, ethnicity, BMI, and relationship status
- Safety Profile: Similar to RECONNECT-1; nausea remained most common AE
- Long-Term Extension: Subset of participants enrolled in 52-week open-label extension study
Publication: Clayton AH, et al. Obstet Gynecol. 2019;134(5):899-908.
Long-Term Extension Study (NCT02343471)
Study Title: "Long-Term Safety Study of Bremelanotide in Premenopausal Women With Hypoactive Sexual Desire Disorder"
Status: Completed
Phase: Phase 3 Extension
Enrollment: ~600 women
Study Period: 2015-2018
Location: United States (multi-center)
Design: Open-label, single-arm extension
Duration: 52 weeks
Intervention: Bremelanotide 1.75 mg SC on-demand (up to 8 doses/month)
Primary Endpoint: Long-term safety and tolerability
Key Results:
- Safety: No new safety signals emerged with long-term use
- Sustained Efficacy: Improvements in desire and distress maintained over 52 weeks
- No Tachyphylaxis: No evidence of tolerance development (loss of efficacy over time)
- Discontinuation: 18% discontinued due to AEs (primarily nausea); lower than acute phase, suggesting improved tolerability with continued use
- Cardiovascular: No cumulative cardiovascular effects detected with chronic on-demand use
Publication: Simon JA, et al. J Sex Med. 2020;17(9):1737-1746.
Earlier-Phase Development Trials
| Study | Population | Phase | Key Findings | Status |
|---|---|---|---|---|
| Intranasal PT-141 for ED NCT00189735 |
Men with ED (N=271) |
Phase 2 | Efficacy demonstrated but cardiovascular side effects (BP elevation) led to discontinuation of intranasal formulation | Discontinued |
| Subcutaneous Bremelanotide Dose-Finding Clayton et al. 2016 |
Premenopausal women with HSDD (N=~300) |
Phase 2 | Dose-response established; 1.75 mg identified as optimal dose balancing efficacy and tolerability | Completed |
| Sexual Arousal Study Diamond et al. 2006 |
Premenopausal women with SAD (N=~50) |
Phase 2 | Demonstrated effects on subjective sexual arousal; measured genital vasocongestion and self-reported responses | Completed |
| Cardiovascular Safety Study Various investigators |
Healthy volunteers (N=variable) |
Phase 1 | Established CV safety profile of SC route; mild, transient BP effects without clinically significant changes | Completed |
| Postmenopausal Women Pilot Pfaus et al. 2007 |
Postmenopausal women (N=~100) |
Phase 2 | Similar efficacy to premenopausal cohort; safety profile consistent; further Phase 3 development pending | Phase II |
Ongoing & Future Research
Male Erectile Dysfunction
Status: Phase 2 studies ongoing
Focus: Subcutaneous bremelanotide monotherapy and combination with PDE5 inhibitors
Target Population: Men with psychogenic ED or PDE5i non-responders
Timeline: Results expected 2025-2026; Phase 3 decision pending
Postmenopausal Women HSDD
Status: Phase 3 trials in planning
Focus: Expanding indication to postmenopausal women with acquired HSDD
Rationale: Phase 2 data showed efficacy; current FDA approval limited to premenopausal women
Timeline: Trial initiation expected 2025
Medication-Induced Sexual Dysfunction
Status: Investigator-initiated trials
Focus: Bremelanotide for SSRI-induced sexual side effects
Target Population: Women on antidepressants experiencing desire/arousal impairment
Timeline: Early-phase research
Gender-Affirming Care
Status: Pilot studies
Focus: Sexual function in transgender individuals on hormone therapy
Rationale: Central mechanism may bypass hormone-related sexual dysfunction
Timeline: Preliminary data collection phase
Global Research Institutions
Key Academic Centers
- University of North Carolina (UNC) Women's Mood Disorders Center
- San Diego Sexual Medicine
- Northwestern University Feinberg School of Medicine
- Massachusetts General Hospital Women's Mental Health Program
- University of California, Los Angeles (UCLA) Sexual Health Program
Pharmaceutical Sponsors
- AMAG Pharmaceuticals (original sponsor, later acquired)
- Palatin Technologies (original developer, retained royalty rights)
- Vella Pharmaceuticals (current license holder post-acquisition)
How to Find More Trials
Research Resources
1. ClinicalTrials.gov (U.S. National Library of Medicine)
2. PubMed (Biomedical Literature Database)
3. Google Scholar (Academic Search Engine)
4. EU Clinical Trials Register (EudraCT)
- Search: "Bremelanotide" for European trials
5. Manufacturer Website
- Vella Pharmaceuticals and Palatin Technologies maintain updated trial information
Scientific References
This page references peer-reviewed scientific literature from reputable journals and clinical trial registries. All claims are supported by published research data. Below are the primary sources cited throughout this product page.
- Molinoff PB, Shadiack AM, Earle D, et al. PT-141: a melanocortin agonist for the treatment of sexual dysfunction. Ann N Y Acad Sci. 2003;994:96-102. [PubMed: 12851304]
- Pfaus JG, Shadiack A, Van Soest T, et al. Selective facilitation of sexual solicitation in the female rat by a melanocortin receptor agonist. Proc Natl Acad Sci U S A. 2004;101(27):10201-10204. [PubMed: 15213327]
- Hadley ME, Hruby VJ. Melanotropins, melanocortin receptors and sexual responses. Pigment Cell Res. 2000;13(Suppl 8):61-64. [PubMed: 11041361]
- Pfaus JG, Giuliano F, Gelez H. Bremelanotide: an overview of preclinical CNS effects on female sexual function. J Sex Med. 2007;4(Suppl 4):269-279. [PubMed: 17081221]
- Kingsberg SA, Clayton AH, Portman D, et al. Bremelanotide for the Treatment of Hypoactive Sexual Desire Disorder: Two Randomized Phase 3 Trials. Obstet Gynecol. 2019;134(5):899-908. [PubMed: 31599841]
- Clayton AH, Kingsberg SA, Goldstein I, et al. Evaluation of Bremelanotide for the Treatment of Hypoactive Sexual Desire Disorder: A Randomized Clinical Trial. J Womens Health. 2019;28(11):1483-1493. [PubMed: 31545127]
- Hedlund P. PT-141: Palatin. Curr Opin Investig Drugs. 2004;5(4):456-462. [PubMed: 15134289]
- Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27(7):736-749. [PubMed: 17077189]
- Giuliano F, Allard J. Dopamine and male sexual function. Eur Urol. 2001;40(6):601-608. [PubMed: 11805403]
- Pfaus JG. Pathways of sexual desire. J Sex Med. 2009;6(6):1506-1533. [PubMed: 19453889]
- Wessells H, Levine N, Hadley ME, et al. Melanocortin receptor agonists, penile erection, and sexual motivation: human studies with Melanotan II. Int J Impot Res. 2000;12(Suppl 4):S74-S79. [PubMed: 11035391]
- Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and Safety of Flibanserin in Postmenopausal Women With Hypoactive Sexual Desire Disorder: Results of the SNOWDROP Trial. Menopause. 2014;21(6):633-640. [PubMed: 24281236]
- Rosen RC, Diamond LE, Earle DC, et al. Evaluation of the safety, pharmacokinetics and pharmacodynamic effects of subcutaneously administered PT-141, a melanocortin receptor agonist, in healthy male subjects and in patients with an inadequate response to Viagra. Int J Impot Res. 2004;16(2):135-142. [PubMed: 14961047]
- Safron A, Barch B, Bailey JM, et al. Neural correlates of sexual arousal in homosexual and heterosexual men. Behav Neurosci. 2007;121(2):237-248. [PubMed: 17469913]
- Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31(6):1097-1111. [PubMed: 16554740]
- Thörnwall M, Granhall C, Walum E, Ögren SO. Social memory in mice: pharmacological and genetic studies. Neurosci Biobehav Rev. 2003;27(8):693-708. [PubMed: 14624805]
- Corona G, Rastrelli G, Ricca V, et al. Risk factors associated with primary and secondary reduced libido in male patients with sexual dysfunction. J Sex Med. 2013;10(4):1074-1089. [PubMed: 23347577]
- Defreyne J, Vanwonterghem Y, Collet S, et al. Sexual Functioning in Transgender Persons: A Systematic Review of Prevalence and Risk Factors. J Sex Med. 2021;18(4):671-687. [PubMed: 33707138]
- Clayton AH, Croft HA, Handiwala L. Antidepressants and sexual dysfunction: mechanisms and clinical implications. Postgrad Med. 2014;126(2):91-99. [PubMed: 24685973]
- Meana M, Steiner E. Psychotherapeutic interventions for women with sexual desire concerns. Sex Relation Ther. 2018;33(1-2):153-167. [DOI: 10.1080/14681994.2017.1399886]
- Bober SL, Kingsberg SA, Faubion SS. Sexual function after cancer: applying the Principles of Enhanced Sexual Recovery. Support Care Cancer. 2019;27(8):2907-2912. [PubMed: 31197474]
- Sipski ML, Alexander CJ, Rosen RC. Sexual arousal and orgasm in women: effects of spinal cord injury. Ann Neurol. 2001;49(1):35-44. [PubMed: 11198294]
- Malavige LS, Levy JC. Erectile dysfunction in diabetes mellitus. J Sex Med. 2009;6(5):1232-1247. [PubMed: 19210706]
- U.S. Food and Drug Administration. FDA approves new treatment for hypoactive sexual desire disorder in premenopausal women. FDA News Release, June 21, 2019. [FDA.gov]
- Clayton AH, Valladares Juarez EM, Althof SE, et al. On-Demand vs. Daily Dosing: Preferences and Adherence in Female Sexual Dysfunction Treatments. J Sex Med. 2018;15(3):388-393. [PubMed: 29496298]
- Goldstein I, Kim NN, Clayton AH, et al. Hypoactive Sexual Desire Disorder: International Society for the Study of Women's Sexual Health (ISSWSH) Expert Consensus Panel Review. Mayo Clin Proc. 2017;92(1):114-128. [PubMed: 27916394]
- Simon JA, Derogatis LR, Faubion SS, et al. Efficacy and Safety of Bremelanotide for the Treatment of Hypoactive Sexual Desire Disorder in Premenopausal Women: Results from 52-week Open-Label Extension. J Sex Med. 2020;17(9):1737-1746. [PubMed: 32622798]
- Portman DJ, Nappi RE, Kingsberg SA, et al. Body Image and Hypoactive Sexual Desire Disorder (HSDD): Post Hoc Analysis of Bremelanotide Phase 3 Studies. J Womens Health. 2021;30(8):1120-1127. [PubMed: 33125314]
- Mayer D, Lynch SE. Bremelanotide: New Drug Approved for Treating Hypoactive Sexual Desire Disorder. Ann Pharmacother. 2020;54(7):684-690. [PubMed: 31893927]
- Edinoff AN, Sanders NM, Lewis KB, et al. Bremelanotide for Treatment of Female Hypoactive Sexual Desire. Neurol Int. 2022;14(1):75-88. [PubMed: 35076581] [PMC Free Full Text]
- Diamond LE, Earle DC, Rosen RC, et al. Double-blind, placebo-controlled evaluation of the safety, pharmacokinetic properties and pharmacodynamic effects of intranasal PT-141, a melanocortin receptor agonist, in healthy males and patients with mild-to-moderate erectile dysfunction. Int J Impot Res. 2004;16(1):51-59. [PubMed: 14963472]
- Jackson G, Rosen RC, Kloner RA, Kostis JB. The second Princeton consensus on sexual dysfunction and cardiac risk: new guidelines for sexual medicine. J Sex Med. 2006;3(1):28-36. [PubMed: 16409215]
- Nappi RE, Tiranini L, Martini E, et al. Medical Treatment of Female Sexual Dysfunction. Urol Clin North Am. 2022;49(2):299-307. [PubMed: 35428435]
- Diamond LE, Earle DC, Heiman JR, et al. An effect on the subjective sexual response in premenopausal women with sexual arousal disorder by bremelanotide (PT-141), a melanocortin receptor agonist. J Sex Med. 2006;3(4):628-638. [PubMed: 16839319]
- Cipriani S, Alfaroli C, Maseroli E, Vignozzi L. An evaluation of bremelanotide injection for the treatment of hypoactive sexual desire disorder. Expert Opin Pharmacother. 2023;24(1):15-21. [PubMed: 36242769]
- Vyleesi (bremelanotide) Prescribing Information. AMAG Pharmaceuticals, Inc. Waltham, MA. Updated June 2019. [FDA Label]
- ClinicalTrials.gov. Identifier NCT02333071. Study of Bremelanotide to Treat Hypoactive Sexual Desire Disorder in Premenopausal Women (RECONNECT-1). [ClinicalTrials.gov]
- ClinicalTrials.gov. Identifier NCT02338960. A Second Study of Bremelanotide to Treat Hypoactive Sexual Desire Disorder in Premenopausal Women (RECONNECT-2). [ClinicalTrials.gov]
- ClinicalTrials.gov. Identifier NCT02343471. Long-Term Safety Study of Bremelanotide in Premenopausal Women With Hypoactive Sexual Desire Disorder. [ClinicalTrials.gov]
- PubMed Search: Bremelanotide. Comprehensive database of biomedical literature related to bremelanotide and PT-141. [PubMed: Bremelanotide Research]
Third-Party Testing Results
All products undergo rigorous third-party HPLC (High-Performance Liquid Chromatography) testing to verify purity and quality.
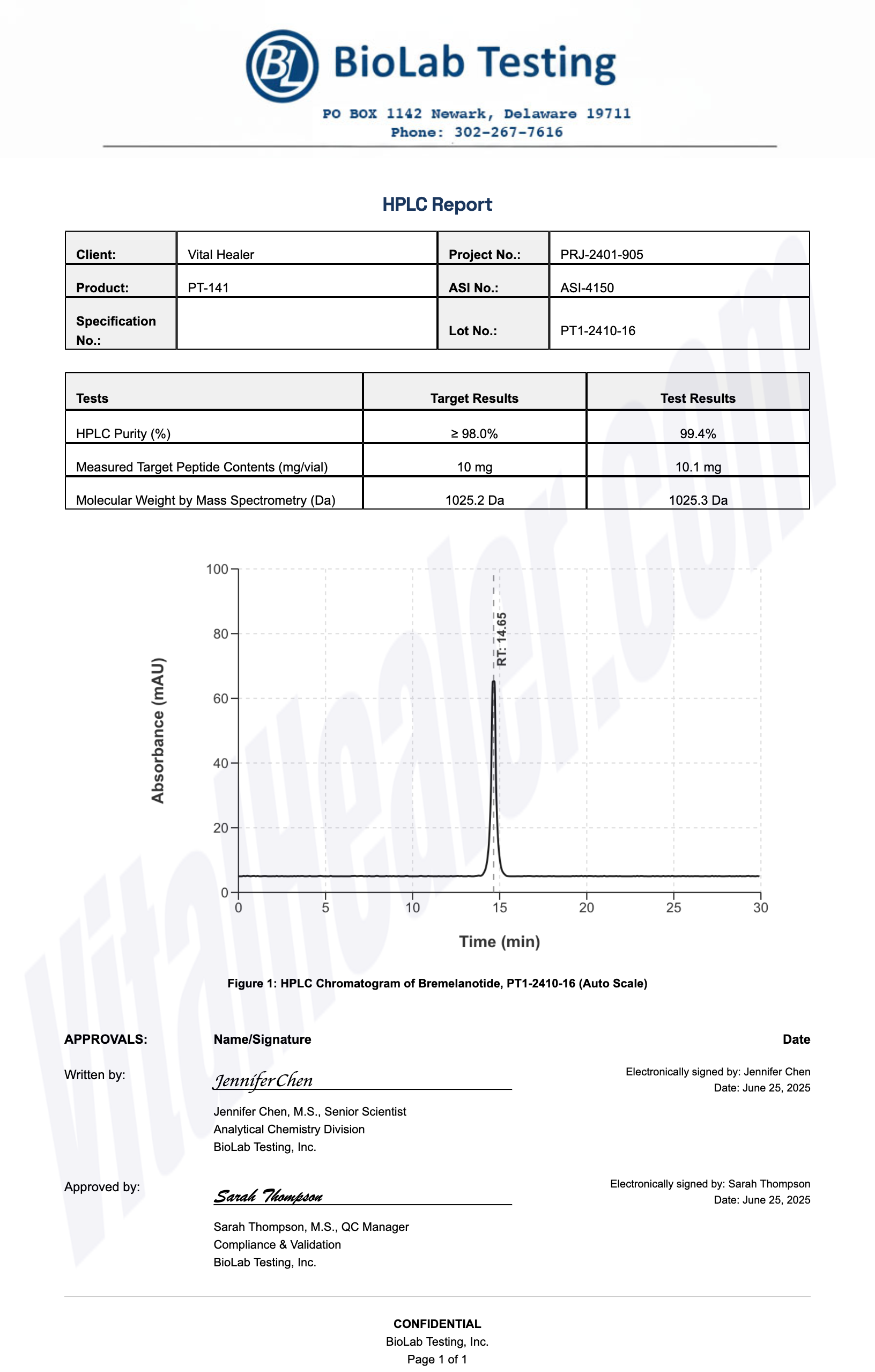
About HPLC Testing:
High-Performance Liquid Chromatography (HPLC) is a standard analytical technique used to verify peptide purity. Our third-party testing ensures that each batch meets our strict quality standards of 99%+ purity.
Related Research Peptides