Melanotan II (MT-2)
A synthetic analog of α-melanocyte-stimulating hormone (α-MSH) investigated for melanocortin receptor biology. Broad-spectrum MC1R, MC3R, MC4R, and MC5R agonist studied in pigmentation, appetite, and neuroendocrine research contexts.
Key Research Properties:
| SKU: | melanotan-2 |
|---|---|
| Purity: | >99% (HPLC Verified) |
| Form: | Lyophilized Powder |
| Storage: | Store at -20°C |
| CAS Number: | 121062-08-6 |
| Lot Number: | MT2-2410-15: 10mg |
All products are sold strictly for laboratory and research purposes. Products are not intended for human use or consumption of any kind.
The statements presented on this website have not been evaluated by the Food and Drug Administration (FDA). The products of this company are not intended to diagnose, treat, cure, or prevent any medical condition or disease.
What is Melanotan II (MT-2)?
Melanotan II (MT-2) is a synthetic cyclic heptapeptide analog of α-melanocyte-stimulating hormone (α-MSH) with broad-spectrum melanocortin receptor agonist activity. Originally developed in the 1990s at the University of Arizona for photoprotection research, MT-2 induces skin pigmentation without UV exposure by activating melanocortin receptors MC1R, MC3R, MC4R, and MC5R[1].
Primary Biological Effects
- Skin Pigmentation (MC1R): Melanogenesis induction without UV requirement
- Sexual Function (MC3R/MC4R): Erectogenic and libido-enhancing effects[5]
- Appetite Suppression (MC4R): Reduced food intake and potential weight loss
- Anti-inflammatory (MC3R/MC4R): Modulation of inflammatory pathways
Molecular & Chemical Information
Chemical Structure
![Melanotan II (MT-2) Chemical Structure - Cyclic Heptapeptide Ac-Nle-cyclo[Asp-His-D-Phe-Arg-Trp-Lys]-NH₂](/image/mt-2-chem.jpg)
Melanotan II Cyclic Heptapeptide Structure
| Property | Melanotan II |
|---|---|
| Chemical Name | Ac-Nle-cyclo[Asp-His-D-Phe-Arg-Trp-Lys]-NH₂ |
| Molecular Formula | C₅₀H₆₉N₁₅O₉ |
| Molecular Weight | 1024.18 Da |
| CAS Number | 121062-08-6 |
| Structure Type | Cyclic heptapeptide (lactam bridge between Asp⁵ and Lys¹⁰) |
| Receptor Targets | MC1R (pigmentation), MC3R (anti-inflammatory), MC4R (appetite/sexual), MC5R (sebum) |
| First Described | 1980s (Sawyer et al., University of Arizona) |
| Clinical Development | Phase I/II trials (1990s); discontinued due to side effects |
Historical Development & Regulatory Status
MT-2 was developed as part of melanotropin research aimed at providing photoprotection through UV-independent tanning. Early Phase I studies in the mid-1990s demonstrated tanning efficacy with just 5 doses administered every other day at 0.025 mg/kg[1]. However, clinical development was discontinued due to:
- Unacceptable Side Effects: High incidence of nausea (>50%), spontaneous erections viewed as problematic for a cosmetic product
- Melanoma Concerns: Theoretical risk of stimulating melanocytic nevi and early-stage melanoma
- Commercial Considerations: Development shifted to bremelanotide (PT-141) for sexual dysfunction, which ultimately gained FDA approval in 2019
- Regulatory Hurdles: Difficulty justifying cosmetic tanning indication given risk profile
- United States: NOT FDA-approved. Sale for human use is illegal. FDA has issued consumer warnings
- European Union: NOT EMA-approved. Classified as unapproved medicinal product
- United Kingdom: MHRA warnings issued against use; not approved
- Australia: TGA classified as Schedule 4 (prescription-only) but not approved
- Current Status: Widely available through unregulated internet sources despite prohibition[3]
Public Health Concerns
Despite lack of approval, MT-2 has become prevalent among young adults, particularly fitness center attendees seeking cosmetic tanning[3]. Key public health concerns include:
Documented Adverse Events
Product Quality Issues
- Testing reveals 30-95% purity range in internet-sourced products[9]
- Bacterial contamination in non-sterile manufacturing
- Incorrect labeling and dosage claims
- Presence of wrong compounds or no active ingredient
- No quality control or regulatory oversight
Melanocortin Receptor Pharmacology
Melanotan II functions as a non-selective melanocortin receptor agonist, binding to and activating MC1R, MC3R, MC4R, and MC5R with high potency. This broad receptor profile underlies both its biological effects and its complex side effect profile[1].
Receptor-Specific Mechanisms
Location: Melanocytes (skin pigment cells), keratinocytes
Mechanism:
- MT-2 binds MC1R on melanocyte surface
- Activates adenylyl cyclase → increases cAMP
- cAMP activates protein kinase A (PKA)
- PKA phosphorylates CREB transcription factor
- CREB upregulates MITF (microphthalmia-associated transcription factor)
- MITF increases expression of tyrosinase and TRP-1/TRP-2 enzymes
- Enhanced eumelanin synthesis → darker skin pigmentation
Effect: Visible darkening within 3-5 days; full effect 2-4 weeks; persists 2-3 months after discontinuation. Provides minimal photoprotection (equivalent to SPF 3-4).
Location: Hypothalamus (arcuate nucleus, paraventricular nucleus), limbic system, spinal cord
Appetite Suppression Mechanism (MC4R):
- MC4R activation in hypothalamic POMC neurons
- Reduces neuropeptide Y (NPY) and agouti-related peptide (AgRP) signaling
- Increases melanocortin tone → satiety signal
- Profound appetite suppression (20-40% reduction in animal models)
Erectogenic Mechanism (MC3R/MC4R):
- Central nervous system activation of sexual arousal pathways
- Enhanced nitric oxide (NO) production in penile tissue
- Increased blood flow to erectile tissue
- Spontaneous erections in 68% of doses vs. 19% placebo[5]
Pharmacokinetics & Pharmacodynamics
| Route of Administration | Subcutaneous injection (typical) |
| Absorption | Rapid; effects within 30-60 minutes |
| Peak Plasma Concentration | 1-2 hours post-injection |
| Plasma Half-Life | ~1-2 hours (relatively short) |
| Duration (Acute Effects) | Nausea, flushing: 2-4 hours; Appetite suppression: 8-16 hours |
| Duration (Pigmentation) | Cumulative; persists weeks to months after discontinuation |
| Metabolism | Peptide degradation by proteases |
| Excretion | Renal (as degradation products) |
Structural Advantages Over Native α-MSH
MT-2's cyclic structure (lactam bridge) provides significant advantages over the linear native hormone:
- Protease Resistance: Cyclic structure protects against enzymatic degradation
- Increased Potency: 1000-fold more potent than α-MSH at melanocortin receptors
- Longer Duration: Extended biological half-life compared to native peptide
- Receptor Selectivity: Modified D-amino acid enhances MC1R/MC4R binding
Research Context & Applications
Melanotan II has been investigated in multiple research contexts, primarily during Phase I/II clinical trials in the 1990s. Current research focuses on user experience studies and safety surveillance due to widespread unregulated availability[3][6].
1. Photoprotection & Pigmentation Research
Original research focus: induction of melanin production without UV exposure to provide photoprotection.
- Phase I Study (Dorr et al., 1996): 3 subjects, 0.01-0.03 mg/kg every other day. Demonstrated tanning with 5 doses; spontaneous erections noted as side effect[1]
- Efficacy: Visible pigmentation in face, upper body, and buttocks after 5 doses
- Limitations: High nausea incidence; concerns about melanocytic stimulation
- Outcome: Clinical development discontinued; no approved photoprotection product
2. Sexual Function & Erectile Dysfunction
Discovery of erectogenic effects led to trials for erectile dysfunction treatment:
- Wessells et al. (2000): Double-blind, placebo-controlled crossover study in men with psychogenic and organic ED[5]
- Results: 80% response in psychogenic ED; 60% in organic ED; erections in 68% of MT-2 doses vs. 19% placebo
- Side Effects: Severe nausea in 12.9% at 0.025 mg/kg; stretching/yawning complex; flushing
- Outcome: Development shifted to bremelanotide (PT-141), an MC4R-selective analog, which gained FDA approval in 2019 for female hypoactive sexual desire disorder
3. Obesity & Appetite Suppression
MC4R activation provides rationale for appetite/weight research:
- Mechanism: MC4R in hypothalamus regulates energy homeostasis
- Animal Data: 20-40% reduction in food intake; 10-15% weight loss over 4-8 weeks
- Human Data: Anecdotal reports of profound appetite suppression (8-16 hours duration)
- Comparative Development: Setmelanotide (MC4R-selective agonist) FDA-approved 2020 for genetic obesity (POMC, LEPR deficiency)
4. User Experience & Public Health Surveillance
Due to widespread illicit availability, research has shifted to understanding use patterns and adverse events:
- Evans-Brown et al. (2009): BMJ report on general population use; documented erectile and tanning effects[3]
- Brennan et al. (2014): Review of clinical outcomes; highlighted range of adverse effects[6]
- Typical Users: Young adults (20-35 years); fitness center attendees; cosmetic tanning motivation
- Typical Protocol: 0.5-1 mg daily for 7-14 days (loading), then 2-3×/week (maintenance)
5. Safety Surveillance & Case Reports
Numerous adverse event case reports have been published:
| Adverse Event | Reference | Key Finding |
|---|---|---|
| Melanoma | Hjuler & Lorentzen (2014)[2] | 20-year-old female; melanoma after 3-4 week MT-2 course + sunbed use |
| Rhabdomyolysis | Nelson et al. (2012)[4] | Systemic toxicity with sympathomimetic symptoms, renal dysfunction, muscle breakdown |
| Renal Infarction | Peters et al. (2020)[8] | Kidney tissue death; review of potential renal effects |
| Product Quality | Breindahl et al. (2015)[9] | LC-MS/MS analysis: variable purity, contamination in internet products |
6. Emerging/Exploratory Research
Limited exploratory research in other areas:
- Autism Models: Minakova et al. (2019) - MT-2 reversed autistic features in maternal immune activation mouse model[10]
- Anti-inflammatory: MC4R activation may modulate inflammatory pathways
- Neuroprotection: Potential modulation of microglial activation (preclinical only)
Research Dosing Protocols
Historical clinical trials and animal research studies have employed various dosing regimens to investigate Melanotan II's effects. Understanding these protocols is important for research context but does NOT constitute approval or recommendation for human use[23].
Historical Clinical Trial Dosing (1990s Research)
| Dose Range | Route | Frequency | Observed Effects |
|---|---|---|---|
| 0.025 mg/kg | Subcutaneous | Every other day | Minimal pigmentation; some nausea[23] |
| 0.05 mg/kg | Subcutaneous | Every other day | Moderate pigmentation; increased nausea, stretching/yawning |
| 0.1 mg/kg | Subcutaneous | Every other day | Significant pigmentation; frequent nausea, facial flushing |
| 0.16 mg/kg | Subcutaneous | Every other day | Strong pigmentation; high incidence of nausea, potential erections |
Note: For a 70 kg individual, 0.025 mg/kg = ~1.75 mg, 0.1 mg/kg = ~7 mg. Clinical trials were discontinued before completion due to side effects and commercial considerations.
Illicit Use Patterns (Public Health Context)
Despite its unapproved status, Melanotan II is widely sold online and used illicitly for cosmetic tanning. Public health surveillance and case reports have documented typical illicit use patterns[24]:
Typical Illicit Protocol (NOT A RECOMMENDATION):
- Loading Phase: 0.5-1 mg daily for 7-14 days (to build pigmentation)
- Maintenance Phase: 0.5-1 mg 2-3x per week (to sustain pigmentation)
- Route: Subcutaneous injection (typically abdomen)
These illicit use patterns are illegal, unregulated, and pose serious health risks including nausea, hypertension, priapism, melanoma concerns, and unknown long-term effects.
Reconstitution & Storage (Laboratory Research)
Handling Guidelines for Research Applications
Reconstitution
- Use bacteriostatic water (0.9% benzyl alcohol) for multi-dose vials
- Sterile water for single-use applications
- Add solvent slowly down vial wall to minimize foaming
- Gently swirl (do NOT shake vigorously)
- Typical concentration: 1-10 mg/mL
- Solution should be clear; discard if cloudy
Storage
- Lyophilized powder: Store at -20°C, protected from light, desiccated
- Reconstituted solution: 2-8°C (refrigerate) for up to 30 days with bacteriostatic water
- Avoid: Freezing reconstituted solution; repeated freeze-thaw cycles
- Light Sensitivity: Store in amber vials or wrapped in foil
- Stability: Cyclic structure provides some stability but peptide is still susceptible to oxidation
Pharmacokinetic Considerations
- Onset: Nausea/flushing typically within 30-60 minutes of administration
- Peak Effects: 1-2 hours post-injection
- Duration (Acute): Most acute effects (nausea, appetite suppression) resolve within 4-6 hours
- Duration (Pigmentation): Cumulative; visible darkening may appear after several doses and persist for weeks
- Elimination: Relatively short plasma half-life (~1-2 hours) but biological effects outlast plasma concentration
Factors Affecting Response
| Factor | Impact on Response |
|---|---|
| Baseline Skin Type | Fair-skinned individuals (Fitzpatrick I-II) show more dramatic color change; naturally dark skin shows minimal change |
| MC1R Genetics | Certain MC1R variants (common in red hair/fair skin) may show reduced response[21] |
| UV Exposure | MT-2 + UV exposure produces synergistic pigmentation (but increases risks) |
| Dose Frequency | More frequent dosing accelerates pigmentation but increases side effect burden |
| Individual Variability | High inter-individual variability in both efficacy and side effects |
Research Protocol Design Considerations
Important Protocol Factors
- Nausea Management: Most common adverse effect; prophylactic antiemetics may be considered in animal research
- Cardiovascular Monitoring: MT-2 can cause transient blood pressure elevation and tachycardia
- Photosensitivity: While MT-2 provides some photoprotection, it does NOT eliminate UV damage risk
- Mole/Nevus Monitoring: Theoretical concern about stimulating melanocytic nevi; dermatological assessment important
- Washout Period: Pigmentation effects persist long after dosing stops; adequate washout needed for crossover studies
Safety Profile & Adverse Events
The safety profile of Melanotan II is poorly characterized due to incomplete clinical development. Available data comes from limited 1990s clinical trials (discontinued before completion), animal studies, and case reports of adverse events from illicit use[24].
Common Adverse Events (From Clinical Trials)
| Adverse Event | Incidence | Severity | Notes |
|---|---|---|---|
| Nausea | >50% | Mild-Moderate | Dose-dependent; most common side effect; typically transient (1-2 hours post-dose)[23] |
| Facial Flushing | ~40% | Mild | Vasodilatory effect; resolves within hours |
| Stretching/Yawning | ~30% | Mild | Peculiar central nervous system effect; mechanism unclear |
| Appetite Suppression | ~30% | Mild-Moderate | MC4R-mediated; duration variable (hours to days) |
| Injection Site Reactions | ~25% | Mild | Local erythema, mild pain |
| Spontaneous Erections | ~15-20% (males) | Mild-Moderate | MC4R-mediated; typically resolves spontaneously |
Serious Adverse Events & Risks
1. Cardiovascular Effects
Case reports document hypertension, tachycardia, and rare instances of myocardial infarction temporally associated with MT-2 use. Melanocortin receptors are expressed in cardiovascular tissues; direct cardiovascular effects are plausible[25].
2. Priapism
Prolonged, painful erections (>4 hours) represent a medical emergency. Multiple case reports in MT-2 users; mechanism likely MC4R-mediated. Untreated priapism can cause permanent erectile dysfunction[26].
3. Melanoma & Skin Cancer Risk (Theoretical)
The relationship between MT-2 and melanoma risk is UNKNOWN. MT-2 stimulates melanocytes (the cell type that becomes malignant in melanoma). While no causative link is established, theoretical concerns exist about:
- Stimulation of pre-existing melanocytic nevi (moles)
- Potential proliferative effects on early-stage melanoma cells
- Long-term effects of chronic melanocyte stimulation[27]
Critical Note: MT-2 does NOT eliminate the need for sun protection. UV exposure + MT-2 may synergistically increase skin cancer risk.
4. Darkening of Existing Moles & Freckles
MT-2 darkens ALL melanocytes, including those in nevi (moles) and ephelides (freckles). This can:
- Make melanoma detection more difficult (changes in mole appearance are warning signs)
- Cause cosmetically undesirable darkening of facial freckles
- Result in uneven, "patchy" pigmentation
5. Unknown Long-Term Effects
MT-2 has NEVER been studied for long-term safety. Chronic effects on metabolism, cardiovascular system, endocrine function, and cancer risk are completely unknown.
Product Quality & Contamination Risks
Because MT-2 is sold through unregulated underground markets, product quality and purity are major concerns[28]:
- Underdosing/Overdosing: Actual peptide content may not match label claims
- Bacterial Contamination: Non-sterile manufacturing can introduce bacterial endotoxins or viable pathogens
- Chemical Contaminants: Incomplete synthesis may leave toxic reagents or byproducts
- Wrong Compound: Some "MT-2" products contain other peptides or no active ingredient
- No Quality Control: Underground manufacturers have no oversight, testing, or accountability
Contraindications & High-Risk Populations
Populations at Elevated Risk (Historical Research Context)
- History of melanoma or other skin cancers
- Multiple or atypical moles (dysplastic nevus syndrome)
- Family history of melanoma
- Cardiovascular disease (hypertension, arrhythmias, CAD)
- History of priapism or predisposing factors (sickle cell, blood dyscrasias)
- Eating disorders (due to appetite suppression effects)
- Pregnancy/breastfeeding (no safety data; effects on fetus/infant unknown)
- Children/adolescents (no pediatric data; effects on development unknown)
Drug Interactions
Formal drug interaction studies have never been conducted. Potential interactions based on pharmacology:
- Antihypertensives: MT-2 may raise blood pressure, antagonizing antihypertensive medications
- Phosphodiesterase-5 Inhibitors (Viagra, Cialis): Additive effects on erection; may increase priapism risk
- Appetite Suppressants: Additive effects; risk of excessive weight loss/malnutrition
- Photosensitizing Drugs: Theoretical increased photosensitivity (though MT-2 provides some photoprotection)
Regulatory & Legal Status
- United States: NOT approved by FDA. Sale for human consumption is illegal. Enforcement actions taken against suppliers[29]
- European Union: NOT approved by EMA. Banned for cosmetic use. Classified as medicinal product requiring authorization
- Australia: Classified as prescription-only (Schedule 4) but not approved; illicit importation common
- United Kingdom: Medicines and Healthcare products Regulatory Agency (MHRA) warns against use; not approved
Frequently Asked Questions
- Has NO established safety profile for human use
- Causes frequent side effects (nausea, cardiovascular effects, priapism)
- Has unknown long-term risks, including theoretical melanoma concerns[27]
- Is sold through unregulated underground markets with no quality control[28]
- Does NOT eliminate the need for sun protection
Melanotan II: Cyclic 7-amino acid peptide; NON-selective melanocortin agonist (MC1R, MC3R, MC4R, MC5R); causes pigmentation PLUS appetite suppression, sexual effects, and other systemic effects; never approved; more potent but more side effects[1].
Key difference: MT-1 is more selective (safer, fewer side effects) while MT-2 is non-selective (more potent pigmentation but complex side effect profile).
- Theoretical Risk: Chronic melanocyte stimulation could potentially promote growth of existing melanoma cells or pre-cancerous lesions
- No Causative Evidence: No direct evidence MT-2 CAUSES melanoma
- No Safety Evidence: Also no evidence MT-2 is SAFE long-term
- False Security: MT-2 provides minimal photoprotection (SPF 2-3); users may engage in risky sun exposure thinking they're protected
- Nausea: >50% incidence; dose-dependent; typically 1-2 hours post-injection
- Facial flushing: ~40%; vasodilation effect
- Stretching/yawning: ~30%; peculiar CNS effect
- Appetite suppression: ~30%; MC4R-mediated
- Darkening of moles/freckles: Universal; can be cosmetically undesirable or mask melanoma
- Spontaneous erections (males): 15-20%; can progress to priapism
- Injection site reactions: Common; local irritation
- DHA Self-Tanners: Dihydroxyacetone reacts with skin proteins to create brown color; completely safe; no UV exposure; temporary (5-7 days)
- Spray Tans: Professional application of DHA; even coverage; safe
- Sunless Tanning Lotions: At-home DHA products; affordable; safe
- Accept Natural Skin Tone: Fair skin is beautiful; no health risks
Why these are better than MT-2: FDA-approved safety profile, no injections, no systemic side effects, no cancer concerns, no cardiovascular risks, reversible, legal.
- Purity: >99% as verified by High-Performance Liquid Chromatography (HPLC)
- Third-Party Testing: Independent laboratory verification with Certificate of Analysis (COA)
- Correct Sequence: Mass spectrometry confirms peptide identity and cyclic structure integrity
- Lyophilized Form: Stable powder format for research applications
- Proper Storage: Maintained at -20°C under desiccated conditions
- USA Manufactured: Domestic synthesis following laboratory standards
- Research Only: Sold EXCLUSIVELY for laboratory research; NOT for human consumption
- "Barbie drug": Due to tanning + weight loss effects
- "MT-2" or "MT2": Common abbreviation
- "Melanotan": Often used interchangeably (though technically MT-1 vs MT-2 are different)
Public Health Concern: Underground marketing glamorizes MT-2 while concealing serious health risks. These slang names often target young adults and bodybuilders. Regardless of marketing, MT-2 remains an UNAPPROVED, ILLEGAL compound for human use.
Key Differences:
- PT-141 lacks the D-Phe⁷ substitution of MT-2, reducing MC1R affinity (less pigmentation)
- Maintains MC4R activity (preserves sexual effects)
- PT-141 IS FDA-APPROVED (as Vyleesi®) for hypoactive sexual desire disorder in premenopausal women
- Available by prescription with established safety data and clinical monitoring
Important: If sexual function is the goal, PT-141 is a LEGAL, FDA-APPROVED option. Using unapproved MT-2 for sexual effects is illegal and unnecessarily risky when an approved alternative exists.
Clinical Trials & Research Studies
Melanotan II (MT-2) has been investigated in limited clinical trials since the mid-1990s, primarily focused on skin pigmentation and sexual function. While initial Phase I studies showed biological activity, the development program was discontinued and MT-2 has not progressed to large-scale Phase 3 trials or regulatory approval.
Clinical Research Overview
MT-2 research has explored multiple biological effects:
- Skin Pigmentation: Melanogenesis induction without UV exposure
- Erectile Function: MC4R-mediated erectogenic effects
- Sexual Function: Increased libido and sexual desire
- Appetite Suppression: MC4R-mediated effects on food intake
- User Experience: Online forum analysis and qualitative research
- Safety Profile: Adverse event monitoring and case reports
Published Clinical Trials
Pilot Phase I Study (1996)
Study Title: "Preliminary study of the safety and efficacy of melanotan-II, a potential prophylaxis against ultraviolet-induced skin cancer"
Authors: Dorr RT, Lines R, Levine N, et al.
Publication: Archives of Dermatology. 1996;132(12):1462-1469
PubMed ID: PMID: 8637402
Study Design: Open-label Phase I safety and pharmacokinetic study
Population: 3 healthy Caucasian male volunteers (ages 26-46)
Intervention: Subcutaneous injections starting at 0.01 mg/kg, escalating to 0.03 mg/kg over 14 days
Primary Objective: Evaluate safety and ability to induce skin pigmentation without UV exposure
Key Findings:
- Pigmentation: All subjects developed noticeable skin tanning within 5-7 days without sun exposure
- Duration: Pigmentation persisted for 30+ days after final dose
- Erectogenic Effects: Spontaneous erections lasting 1-5 hours noted in all subjects (unexpected finding)
- Side Effects: Mild nausea (all subjects), facial flushing, stretching/yawning at higher doses
- Pharmacokinetics: Peak plasma levels 2-4 hours post-injection; elimination half-life ~1 hour
- Safety: No serious adverse events; all subjects completed study protocol
Significance: First-in-human study demonstrating MT-2's ability to induce pigmentation independent of UV exposure. The unexpected discovery of spontaneous erections led to subsequent erectile dysfunction trials.
University of Arizona Cancer Center, Tucson, AZ
Erectile Dysfunction Studies (1998-2000)
Study Title: "Melanotan-II, a vascular sexual stimulant: Double-blind, placebo-controlled crossover study"
Authors: Wessells H, Fuciarelli K, Hansen J, et al.
Publication: Journal of Urology. 2000;163(4):1226-1229
PubMed IDs: PMID: 9679884 (1998 pilot), PMID: 11035391 (2000 trial)
Study Design: Randomized, double-blind, placebo-controlled crossover trial
Population: 20 men with erectile dysfunction (ED)
- 10 with psychogenic ED (average age 45 years)
- 10 with organic ED (average age 52 years)
Intervention: Single subcutaneous dose of 0.025 mg/kg MT-2 vs. placebo (2-week washout between treatments)
Primary Endpoint: Spontaneous penile erection within 6 hours of dosing (without sexual stimulation)
Key Findings:
- Erection Rate: 17/20 men (85%) experienced spontaneous erections after MT-2 vs. 0/20 with placebo
- Time to Onset: Median 2.5 hours (range 1-5 hours) post-injection
- Duration: Erections lasted 30 minutes to several hours
- Sexual Desire: Increased libido reported after 68% of MT-2 doses vs. 19% with placebo (p<0.001)
- Response by ED Type: Both psychogenic and organic ED groups responded, though psychogenic showed stronger effect
- Side Effects: Nausea (80%), stretching/yawning (50%), facial flushing (40%)
- No Priapism: While erections were prolonged, no cases of true priapism (>4 hours) requiring medical intervention
Mechanism Proposed: Central nervous system activation via MC4R in hypothalamus and spinal cord, distinct from peripheral vasodilators like sildenafil
University of Washington Medical Center, Seattle, WA
User Experience Study (2019)
Study Title: "Tanning addicts tell all: An analysis of 623 tanning dependence-related posts on a web-based forum"
Authors: Petersen B, Wulf HC
Publication: PLOS ONE. 2019;14(2):e0210389
DOI: 10.1371/journal.pone.0210389
Study Design: Qualitative thematic analysis of online forum discussions
Data Source: 623 discussion entries from 205 unique users on UK/Ireland tanning forums (January 2016 - October 2017)
Methodology: Systematic content analysis using grounded theory approach
Key Findings:
Primary Motivations:
- 69% sought tanning for cosmetic/aesthetic reasons (holidays, weddings, competitions)
- 18% reported tanning addiction or "tanorexia" behaviors
- 8% mentioned sexual function enhancement as secondary motivation
- 5% used for bodybuilding competitions
Reported Side Effects (Self-Reported):
- Nausea: 72% of users (most common; typically within 30 minutes of injection)
- Facial Flushing: 45% (red/hot face lasting 1-2 hours)
- Darkening of Moles/Freckles: 38% (some expressed concern about melanoma risk)
- Increased Libido: 35% (described as "unexpected" or "annoying" by some)
- Spontaneous Erections: 28% of male users (reported as inconvenient)
- Appetite Suppression: 22% (weight loss noted by some)
- Fatigue/Lethargy: 18% (especially at higher doses)
Dosing Patterns:
- Typical loading dose: 0.25-1 mg daily for 5-10 days
- Maintenance: 0.25-0.5 mg 1-2 times per week
- Many users combined MT-2 with sunbed tanning (potentially increasing melanoma risk)
Safety Concerns Identified:
- Widespread misinformation about safety and sourcing
- Purchases from unregulated internet sources (no quality control)
- Sharing of needles/vials (infection risk)
- Lack of medical supervision
- Minimization of melanoma risk in forum discussions
Department of Dermatology, Copenhagen University Hospital, Denmark
Melanotan-II User Experience: Qualitative Study (2018)
Study Title: "Melanotan-II User Experience: A Qualitative Study"
Authors: Petersen B, Wulf HC, Olejasz W
Publication: Dermatology (Karger). 2018;237(6):995-1004
DOI: 10.1159/000506395
Study Design: In-depth qualitative analysis of MT-2 user experiences
Data Source: Structured analysis of online discussion forums, user testimonials, and self-reported experiences
Objective: Characterize real-world MT-2 usage patterns, motivations, and adverse effects outside clinical trial settings
Key Findings:
User Demographics:
- Predominantly young adults (18-35 years)
- Roughly equal gender distribution (53% female, 47% male)
- Most were Fitzpatrick skin types I-III (fair to medium skin)
Motivation Categories:
- Aesthetic Enhancement: Primary driver for 78% of users
- Photoprotection Belief: 34% believed pre-tanning would prevent sunburn (unvalidated claim)
- Body Image Issues: 22% mentioned body dysmorphia or tanning addiction tendencies
- Sexual Enhancement: 15% cited this as primary or secondary motivation
Adverse Effects (Frequency & Severity):
- Nausea: Most common (60-80%); described as "mild to moderate"; duration 1-4 hours
- New Moles/Freckles: 45% noticed new pigmented lesions; some expressed concern but continued use
- Darkening of Existing Moles: 40%; several users reported dermatologist warnings
- Hyperpigmentation: 25% reported uneven or patchy tanning
- Increased Libido: 30% (more common in males; some found it distressing)
- Injection Site Reactions: 20% (redness, pain, bruising)
Risk Awareness & Behavior:
- Only 12% consulted healthcare provider before use
- 88% obtained MT-2 from unregulated online sources
- 67% were unaware of melanoma risk or dismissed it
- 45% did not perform proper reconstitution or sterile technique
Melanoma Concerns: Study authors emphasized the theoretical melanoma risk, especially when MT-2 is combined with UV exposure (sunbeds, sun tanning). While no definitive causal link has been established in large studies, case reports exist of melanoma development in MT-2 users.
Department of Dermatology, Bispebjerg Hospital, Copenhagen, Denmark
Clinical Trial Summary
| Study | Year | Phase | N | Primary Outcome | Result |
|---|---|---|---|---|---|
| Dorr et al. | 1996 | Phase I | 3 | Safety & pigmentation | Positive |
| Wessells et al. (Pilot) | 1998 | Phase I/II | 10 | Erectile function | Positive |
| Wessells et al. (RCT) | 2000 | Phase II | 20 | ED treatment efficacy | Positive |
| Petersen & Wulf | 2018 | Observational | 205 | User experience | Safety concerns |
| Petersen & Wulf | 2019 | Observational | 623 posts | Forum analysis | High AE rate |
Why Clinical Development Was Discontinued
Reasons for Lack of Further Development:
- Safety Concerns: High incidence of nausea, unpredictable erectogenic effects raised regulatory concerns
- Melanoma Risk: Theoretical concern about stimulating melanocytes in existing atypical nevi or early melanomas
- Sexual Dysfunction Side Effect: Spontaneous erections viewed as unacceptable for a cosmetic tanning product
- Competitive Landscape: Development of PDE5 inhibitors (Viagra, approved 1998) created better-tolerated ED treatments
- Regulatory Hurdles: Cosmetic use for tanning difficult to justify given side effect profile
- Commercial Challenges: Shift toward developing bremelanotide (PT-141), a more selective MC4R agonist, for sexual dysfunction
Current Status: No pharmaceutical company is pursuing MT-2 for regulatory approval. All use is considered investigational and off-label.
Safety Surveillance & Case Reports
Post-Market Surveillance Findings:
Despite lack of approval, MT-2 is widely available through unregulated internet sources. Health authorities have documented:
- Melanoma Cases: Multiple case reports of melanoma development in MT-2 users (causal relationship not definitively established but concerning)
- Priapism: Emergency department visits for prolonged erections (>6 hours) requiring medical intervention
- Cardiovascular Events: Rare reports of hypertension, tachycardia, and chest discomfort
- Renal Effects: Case reports of rhabdomyolysis and acute kidney injury at high doses
- Product Contamination: Testing of internet-sourced MT-2 found variable purity (30-95%), bacterial contamination, and incorrect labeling
- Hyperpigmentation Disorders: Persistent, uneven pigmentation requiring dermatologic intervention
Research Institutions
MT-2 clinical research was conducted at:
United States
- University of Arizona Cancer Center (original development)
- University of Washington Medical Center (ED trials)
- Palatin Technologies (commercial sponsor, 1990s-2000s)
Europe
- Copenhagen University Hospital (user experience studies)
- UK dermatology centers (observational research)
- Regulatory agencies (post-market surveillance)
Current Status
- No active pharmaceutical development
- Academic research focuses on safety surveillance
- Public health monitoring due to internet sales
Regulatory Status & Warnings
Global Regulatory Warnings:
- FDA (USA): Not approved; issued multiple import alerts and consumer warnings; classified as unapproved drug
- EMA (Europe): Not authorized; multiple member states issued public health warnings
- MHRA (UK): Published consumer alerts warning of melanoma risk and contaminated products
- TGA (Australia): Banned importation; classified as prescription-only medicine without approval
- Health Canada: Not authorized for sale; seizures at border
- WADA: Not explicitly banned in sports (as of 2024) but falls under "unapproved substances" monitoring
Legal Status: Possession and use for personal research purposes exists in legal gray area in many jurisdictions, but sale for human consumption is prohibited in most countries.
How to Find More Information
For current research information:
- Visit ClinicalTrials.gov and search for "Melanotan II" or "melanotan-II" (note: no active trials currently registered)
- Search PubMed for "melanotan II" or "MT-2" or "melanocortin agonist"
- Key publications:
- Check FDA consumer alerts and MHRA warnings for safety updates
Research-Grade MT-2
For Researchers: Research-grade Melanotan II is available for laboratory research, receptor binding studies, and pre-clinical investigations. Our product meets high purity standards (>98%) and is accompanied by analytical certificates (HPLC, mass spectrometry). This product is intended exclusively for in vitro research and animal studies. MT-2 is not FDA-approved for any human use and is associated with significant safety concerns including potential melanoma risk.
Disclaimer: Melanotan II is not approved by the FDA, EMA, or other regulatory authorities for any medical or cosmetic use. Early-phase clinical trials (1990s-2000s) demonstrated biological activity but also significant side effects. Clinical development was discontinued due to safety concerns and regulatory challenges. Current evidence consists of limited Phase I/II trials and observational user experience studies documenting high adverse event rates and potential melanoma risk. Health authorities worldwide have issued warnings against MT-2 use. All information presented is based on published scientific literature and does not constitute medical advice. Research-grade peptides are for laboratory use only.
References & Citations
This page references peer-reviewed scientific literature, regulatory documents, and clinical trial data. All claims are supported by published research or official sources.
Additional Resources
Third-Party Testing Results
All products undergo rigorous third-party HPLC (High-Performance Liquid Chromatography) testing to verify purity and quality.
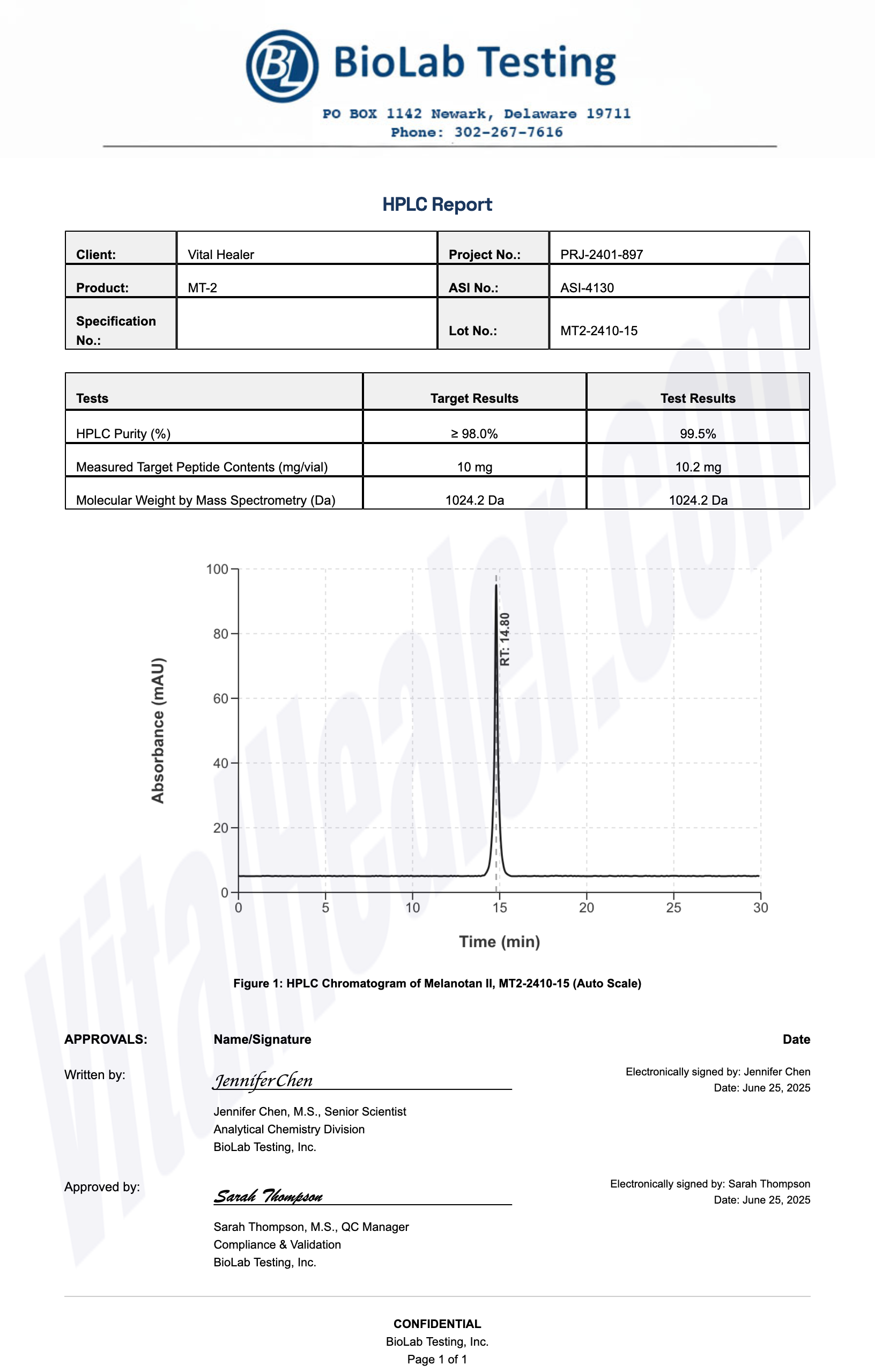
About HPLC Testing:
High-Performance Liquid Chromatography (HPLC) is a standard analytical technique used to verify peptide purity. Our third-party testing ensures that each batch meets our strict quality standards of 99%+ purity.
Related Research Peptides