Oxytocin Peptide
A nine-amino acid neuropeptide hormone investigated for its role in social behavior, stress response, maternal function, and neuroendocrine regulation. Often called the "social hormone," oxytocin modulates trust, empathy, bonding, and emotional processing.
Key Research Properties:
| SKU: | oxytocin |
|---|---|
| Purity: | >99% (HPLC Verified) |
| Form: | Lyophilized Powder |
| Storage: | Store at -20°C |
| CAS Number: | 50-56-6 |
| Lot Number: | OXY-2410-11: 10mg |
All products are sold strictly for laboratory and research purposes. Products are not intended for human use or consumption of any kind.
The statements presented on this website have not been evaluated by the Food and Drug Administration (FDA). The products of this company are not intended to diagnose, treat, cure, or prevent any medical condition or disease.
What is Oxytocin?
Oxytocin is a small, nine-amino acid peptide hormone synthesized and released primarily in the brain. This neuropeptide has properties and functions essential to mammalian behavior, social cognition, stress regulation, and reproductive physiology.
Research interest in oxytocin has increased exponentially over the last two decades. At present, approximately 30,000 research papers listed on PubMed investigate oxytocin in some capacity[ 2]. While often informally called the "love hormone" or "social hormone," these labels are oversimplifications that do not accurately reflect the biological complexity of oxytocin's actions across different contexts and species[6].
Molecular & Chemical Information
| Molecular Formula | C₄₃H₆₆N₁₂O₁₂S₂ |
|---|---|
| Molecular Weight | 1007.19 g/mol |
| Sequence | Cys-Tyr-Ile-Gln-Asn-Cys-Pro-Leu-Gly-NH₂ |
| Structure Type | Cyclic nonapeptide (9 amino acids) |
| Disulfide Bridge | Between Cys¹ and Cys⁶ (critical for activity) |
| CAS Number | 50-56-6 |
| Half-Life | ~3-5 minutes (intravenous) |
Structural Significance:
The disulfide bridge between the two cysteine residues is essential for oxytocin's biological activity. This cyclic structure stabilizes the peptide and is required for binding to the oxytocin receptor (OTR). The C-terminal glycine is amidated (Gly-NH₂), which is also critical for receptor activation.
Evolutionary Conservation
The oxytocin/vasopressin signaling system dates back more than 600 million years. Oxytocin/vasopressin-like peptides have been identified in numerous invertebrate phyla including molluscs, annelids, nematodes, and insects[15]. This ancient lineage suggests fundamental importance in regulating reproductive and social behaviors across evolutionary time.
Physiological Context
Oxytocin is synthesized in magnocellular neurons of the paraventricular nucleus (PVN) and supraoptic nucleus (SON) of the hypothalamus. It is transported via axonal projections to the posterior pituitary, where it is stored and released into systemic circulation[1].
Additionally, oxytocin is released centrally within the brain, acting as a neuromodulator in regions including the amygdala, hippocampus, nucleus accumbens, and olfactory bulb. This dual system—peripheral hormonal release and central neuromodulation—allows oxytocin to coordinate physiological and behavioral responses simultaneously[2].
Research Use Only
This product is sold strictly for in-vitro laboratory research by qualified professionals. Oxytocin is NOT approved by the FDA for human use outside of specific pharmaceutical formulations (Pitocin) administered in clinical settings. Research-grade oxytocin is not for human consumption, self-administration, or veterinary use.
Quality Assurance & Testing
Every batch of our oxytocin undergoes rigorous third-party testing to ensure pharmaceutical-grade quality:
Purity
Verified by HPLC
Identity Confirmed
Mass Spectrometry
Party Tested
Independent Lab
- HPLC (High-Performance Liquid Chromatography): Verifies purity and concentration
- Mass Spectrometry: Confirms molecular weight and peptide identity
- Amino Acid Analysis: Validates complete sequence accuracy
- Endotoxin Testing: Ensures levels are within acceptable limits
- Sterility Testing: Confirms absence of microbial contamination
Mechanism of Action
Oxytocin exerts its effects primarily through activation of the oxytocin receptor (OTR), a G-protein coupled receptor (GPCR) widely distributed throughout the brain and peripheral tissues. The mechanisms underlying oxytocin's diverse physiological and behavioral effects are complex and context-dependent.
The Oxytocin Receptor (OTR)
The oxytocin receptor is a seven-transmembrane domain GPCR coupled primarily to Gq/11 proteins. Upon oxytocin binding, the receptor activates phospholipase C (PLC), leading to production of inositol trisphosphate (IP3) and diacylglycerol (DAG), which mobilize intracellular calcium and activate protein kinase C (PKC), respectively[9].
Receptor Distribution:
OTRs are expressed in the uterus, mammary glands, brain (amygdala, nucleus accumbens, olfactory bulb, hippocampus), heart, vasculature, kidney, pancreas, and adipose tissue. This widespread distribution allows oxytocin to coordinate multisystem physiological and behavioral responses.
Central Nervous System Modulation
Within the brain, oxytocin acts as a neuromodulator, influencing neurotransmitter release and neural circuit activity. Key mechanisms include:
- GABAergic Modulation: Oxytocin can reduce inhibitory GABA transmission in certain brain regions, particularly the central amygdala, reducing fear and anxiety responses[3].
- Dopaminergic Enhancement: Oxytocin facilitates dopamine release in the nucleus accumbens and ventral tegmental area (VTA), which is critical for reward processing and motivational aspects of social behavior[5].
- Olfactory Processing: Oxytocin modulates olfactory bulb activity, enhancing social odor recognition and discrimination[7].
- Amygdala Regulation: By reducing amygdala activation to threatening stimuli, oxytocin promotes approach behavior and social engagement[3].
Peripheral Physiological Effects
In peripheral tissues, oxytocin's mechanisms are well-characterized:
Oxytocin binds to OTRs on uterine smooth muscle, triggering calcium influx and myosin light chain phosphorylation, leading to rhythmic contractions. OTR expression dramatically increases during late pregnancy and labor[1].
Suckling stimulates oxytocin release, which acts on myoepithelial cells surrounding mammary alveoli, causing contraction and milk ejection into ducts[1].
Neuroimmune Interactions
Recent research highlights oxytocin's role in modulating immune function and inflammation:
- Oxytocin has anti-inflammatory effects, reducing pro-inflammatory cytokines (TNF-α, IL-6, IL-1β)
- Regulates microglial activation in the brain[2]
- Influences gut microbiota composition, which in turn modulates oxytocin signaling
- Coordinates behavioral and immune consequences attributed to the gut-brain axis
Context-Dependent Effects:
Oxytocin's effects are highly context-dependent and influenced by factors including sex, developmental stage, social environment, baseline anxiety levels, and concurrent hormone levels (e.g., estrogen, testosterone, cortisol). This context-dependency explains why oxytocin can produce seemingly contradictory effects in different experimental paradigms[2].
Research Applications
Oxytocin has been extensively studied across multiple research domains, from social neuroscience to reproductive physiology to psychiatry. The following represents documented research areas supported by peer-reviewed literature.
Oxytocin is widely studied for its role in social cognition, trust, empathy, and attachment[3]. Research applications include:
- Pair Bonding: Studies in prairie voles demonstrate oxytocin's necessity for partner preference formation and monogamous pair bonding
- Parent-Infant Attachment: Maternal oxytocin facilitates caregiving behaviors and mother-infant synchrony[13]
- Social Recognition: Oxytocin knockout mice exhibit deficits in social memory and recognition
- Prosocial Behavior: Intranasal oxytocin administration enhances cooperation, generosity, and trust in experimental economic games[4]
Oxytocin's role in social function has led to investigation in conditions characterized by social deficits[14]:
- Autism Spectrum Disorder (ASD): Some studies report improved social cognition with intranasal oxytocin, though results are mixed
- Schizophrenia: Investigation as adjunctive treatment for negative symptoms and social withdrawal
- Social Anxiety Disorder: Research on oxytocin's anxiolytic effects in social contexts[3]
- Depression: Studies examining oxytocin as potential treatment for anhedonia and social withdrawal[19]
- Prader-Willi Syndrome: Clinical trials investigating oxytocin for hyperphagia and social deficits[14]
Emerging research investigates oxytocin's potential in addiction treatment[17]:
- Alcohol Use Disorder: Preclinical studies show oxytocin reduces alcohol self-administration and withdrawal symptoms
- Opioid Addiction: Research on oxytocin for reducing cravings and promoting abstinence
- Stimulant Addiction: Investigation of oxytocin's effects on methamphetamine and cocaine-seeking behavior
- Social Reward Substitution: Hypothesis that oxytocin enhances social rewards, providing alternative to drug rewards
Oxytocin's role in reproduction and maternal behavior is extensively studied[1]:
- Labor and Delivery: Investigation of optimal oxytocin dosing regimens for labor induction
- Postpartum Depression: Research on relationship between oxytocin dysregulation and PPD[19]
- Breastfeeding: Studies on oxytocin's role in milk ejection reflex and maternal-infant bonding
- Maternal Behavior: Preclinical research on oxytocin's necessity for normal caregiving[13]
Oxytocin exhibits analgesic properties in preclinical models[11]:
- Visceral Pain: Oxytocin receptor agonists developed for treatment of abdominal pain and IBS
- Inflammatory Pain: Anti-inflammatory and analgesic effects in models of arthritis and inflammation
- Headache: Investigation in migraine and cluster headache models
Oxytocin influences cardiovascular function through multiple mechanisms:
- Cardiac Regeneration: Studies show oxytocin promotes cardiomyocyte differentiation from progenitor cells
- Blood Pressure Regulation: Investigation of oxytocin's hypotensive effects
- Cardioprotection: Research on oxytocin's protective effects against ischemia-reperfusion injury
Research Dosing Guidelines
The following information is provided for research reference only. All dosing should be determined by qualified researchers based on specific research protocols and regulatory requirements.
Critical Safety Notice
This product is FOR RESEARCH USE ONLY. Not for human consumption or self-administration. Oxytocin administration outside of approved clinical settings is illegal and potentially dangerous.
Reconstitution Instructions
- Use bacteriostatic water or sterile water for injection
- Add solvent slowly down the side of the vial to avoid foaming
- Do not shake; gently swirl or allow to dissolve naturally
- For a 2mg vial: Add 2mL water for 1mg/mL concentration
- For a 5mg vial: Add 5mL water for 1mg/mL concentration
- Use immediately after reconstitution for optimal stability
Storage Requirements
| State | Temperature | Duration |
|---|---|---|
| Lyophilized (powder) | -20°C (freezer) | 2-3 years |
| Lyophilized (short-term) | 2-8°C (refrigerator) | Up to 6 months |
| Reconstituted | 2-8°C (refrigerator) only | 24-48 hours maximum |
Stability Considerations:
- Oxytocin is relatively unstable in solution due to the disulfide bridge
- Avoid repeated freeze-thaw cycles
- Protect from light and excessive heat
- pH should be maintained between 3.0-5.0 for optimal stability
- Reconstituted solutions should be used within 24 hours when possible
Research Protocol Considerations
Published research protocols vary significantly based on study objectives, species, and administration route:
| Administration Route | Typical Research Dose Range | Notes |
|---|---|---|
| Intranasal (Human Studies) | 24-40 IU (single dose) | Most common route in human behavioral research[16] |
| Intravenous (Clinical) | Variable infusion rates | Used in obstetric settings; highly controlled |
| Subcutaneous (Rodent) | 0.1-1.0 mg/kg | Common in preclinical behavioral studies |
| Intraperitoneal (Rodent) | 0.5-10 mg/kg | Wide range depending on behavioral paradigm |
| Intracerebroventricular | Nanogram to microgram range | Direct CNS delivery in animal models |
All products are sold strictly for laboratory and research purposes. Products are not intended for human use or consumption of any kind.
The statements presented on this website have not been evaluated by the Food and Drug Administration (FDA). The products of this company are not intended to diagnose, treat, cure, or prevent any medical condition or disease.
Safety & Side Effects
Oxytocin has a well-established safety profile in clinical obstetric settings where it has been used for decades. However, research into intranasal and other novel administration routes is ongoing.
Clinical Safety Profile
Pharmaceutical oxytocin (Pitocin) is FDA-approved for labor induction and control of postpartum bleeding[1]. When administered appropriately in clinical settings, it is generally well-tolerated. However, improper administration can lead to serious adverse effects.
Common Side Effects (Clinical Use)
- Nausea and vomiting
- Headache
- Tachycardia (rapid heart rate)
- Hypotension (low blood pressure)
- Uterine hyperstimulation (excessive contractions)
Serious Adverse Events (Rare, Clinical Use)
- Water intoxication and hyponatremia (due to antidiuretic effects at high doses)
- Uterine rupture (in cases of excessive dosing or contraindicated use)
- Fetal distress from abnormal uterine contractions
- Cardiac arrhythmias
- Anaphylaxis (extremely rare)
Research Observations (Intranasal Administration)
Most human research studies use intranasal oxytocin at doses of 24-40 IU[16]. Reported side effects in research settings are generally mild:
- Mild nasal irritation
- Headache
- Dizziness
- Flushing
- Fatigue
- Mood changes
- Increased urination
- Gastrointestinal discomfort
Contraindications & Precautions
Absolute Contraindications (Clinical):
- Pregnancy (outside of labor induction under medical supervision)
- Hypersensitivity to oxytocin
- Unfavorable fetal positioning or presentation
- Cephalopelvic disproportion
- Fetal distress
Research Considerations:
- Cardiovascular Conditions: Caution in research involving subjects with hypertension or cardiac disease
- Psychiatric Conditions: Mixed effects observed; careful monitoring required
- Medication Interactions: May interact with prostaglandins, beta-blockers, and other vasoactive drugs
- Pregnancy/Lactation: Contraindicated in research settings
The "Dark Side" of Oxytocin
Recent research has revealed that oxytocin's effects are not universally prosocial. Oxytocin can also facilitate:
- In-Group Favoritism: Enhanced preference for one's own group, potentially at the expense of out-groups[18]
- Envy and Schadenfreude: In competitive contexts, oxytocin may enhance negative emotions toward competitors
- Defensive Aggression: Protection of in-group members can manifest as aggression toward perceived threats
- Social Memory of Negative Events: Enhanced memory for social betrayals or negative social experiences
These findings underscore that oxytocin's effects are highly context-dependent and not simply "pro-social" in all circumstances[18].
Long-Term Safety Considerations
Research Gaps:
There is limited data on the long-term effects of repeated oxytocin administration, particularly via intranasal route. Key questions include receptor desensitization, potential tolerance development, effects on endogenous oxytocin production, and long-term behavioral/physiological consequences. These remain active areas of investigation.
Frequently Asked Questions
Oxytocin is a nine-amino acid neuropeptide hormone synthesized and released primarily in the brain. It plays key roles in social behavior, stress response, reproduction, lactation, and neuroendocrine function[6].
While often called the "love hormone" or "social hormone," these are oversimplifications. Oxytocin's effects are highly context-dependent and influenced by numerous factors including sex, developmental stage, social environment, and concurrent hormones[2].
Yes, but only in specific pharmaceutical formulations for clinical use.
Pharmaceutical oxytocin (brand name Pitocin) is FDA-approved for:
- Labor induction and augmentation
- Control of postpartum hemorrhage
However, research-grade oxytocin sold for laboratory use is NOT for human consumption or medical use. Self-administration of research-grade oxytocin is illegal and potentially dangerous.
Oxytocin is a cyclic nonapeptide (9 amino acids) with the sequence:
Cys-Tyr-Ile-Gln-Asn-Cys-Pro-Leu-Gly-NH₂
- Molecular Formula: C₄₃H₆₆N₁₂O₁₂S₂
- Molecular Weight: 1007.19 g/mol
- Critical Feature: Disulfide bridge between the two cysteine residues (Cys¹-Cys⁶), essential for biological activity
- C-Terminal: Amidated glycine (Gly-NH₂), also critical for receptor activation
Lyophilized (powder) form:
- Long-term: -20°C (freezer) for 2-3 years
- Short-term: 2-8°C (refrigerator) for up to 6 months
After reconstitution:
- Must be refrigerated at 2-8°C
- Use within 24-48 hours maximum
- Protect from light
- Do NOT freeze reconstituted solution
- Avoid repeated freeze-thaw cycles of powder
Oxytocin is one of the most extensively studied neuropeptides, with approximately 30,000 research papers on PubMed[2]. Major research domains include:
- Social Behavior: Trust, empathy, bonding, social recognition[3]
- Reproductive Physiology: Labor, lactation, maternal behavior[1]
- Psychiatry: Autism, social anxiety, depression, schizophrenia[14]
- Addiction: Alcohol, opioids, stimulants[17]
- Pain: Analgesia, visceral pain, inflammation[11]
- Stress & Emotion: Anxiety, fear, stress response[3]
See the "References & Citations" tab for detailed bibliography.
NO. This product is FOR RESEARCH USE ONLY.
Research-grade oxytocin is not approved by the FDA for human consumption, medical use, or veterinary applications outside of approved pharmaceutical formulations. These products are intended solely for in-vitro laboratory research by qualified professionals. Any other use is strictly prohibited by law.
Our oxytocin is guaranteed to be 99%+ pure, verified through:
- HPLC (High-Performance Liquid Chromatography): Confirms purity level
- Mass Spectrometry: Verifies molecular weight and identity
- Amino Acid Analysis: Validates sequence accuracy
Each batch comes with third-party testing certificates available upon request.
This is an ongoing debate in the field. Evidence suggests:
- Yes: Some studies show behavioral and neural effects consistent with central action; intranasal administration can deliver peptides to the brain via olfactory and trigeminal nerve pathways
- Uncertain: The amount reaching the brain may be small; some effects may be mediated by peripheral receptors or via vagal nerve pathways
- Alternative Explanation: Effects may result from peripheral absorption and indirect modulation of brain function
This remains an active area of research with no definitive consensus[16].
Clinical Trials
Oxytocin is one of the most extensively studied neuropeptides in both basic neuroscience and clinical research. As an endogenous hormone with diverse physiological and behavioral effects, oxytocin has been investigated in hundreds of clinical trials spanning autism spectrum disorder, psychiatric conditions, social cognition, obstetric applications, and metabolic disorders.
Autism Spectrum Disorder (ASD) Research
Intranasal oxytocin has emerged as one of the most promising pharmacological interventions for social deficits in autism spectrum disorder, with multiple Phase 2 and Phase 3 trials completed or ongoing worldwide.
Intranasal Oxytocin for ASD Social Deficits (NCT01944046)
Study Title: "Oxytocin Treatment for Social Deficits in Youth With Autism Spectrum Disorders"
Status: Completed
Phase: Phase 2
Enrollment: 32 children and adolescents with ASD
Study Period: 2013-2016
Location: Stanford University, USA
Design: Randomized, double-blind, placebo-controlled crossover
Duration: 6 weeks per treatment arm
Intervention: Intranasal oxytocin 24 IU twice daily vs. placebo
Population: Ages 6-17, diagnosis of ASD
Key Results:
- Primary Outcome (Social Responsiveness Scale): Significant improvement in parent-rated social responsiveness with oxytocin vs. placebo (p=0.02)
- Subgroup Analysis: Greater effects in children with lower baseline oxytocin levels
- Functional Improvements: Enhanced eye contact, reduced anxiety in social situations
- Safety: Well tolerated; no serious adverse events; mild nasal irritation most common (15%)
- Duration Effects: Benefits emerged after 4 weeks of daily administration
Publication: Parker KJ, et al. Proc Natl Acad Sci U S A. 2017;114(30):8119-8124.
OTAP (Oxytocin Treatment for Autism in Preschoolers) Trial
Study Title: "Intranasal Oxytocin Treatment for Core Social Deficits in Autism Spectrum Disorder - Early Intervention"
Status: Completed
Phase: Phase 2
Enrollment: 48 preschool children with ASD
Study Period: 2018-2021
Design: Randomized, double-blind, placebo-controlled
Duration: 12 weeks
Intervention: Weight-based intranasal oxytocin dosing vs. placebo
Population: Ages 3-5, early ASD diagnosis
Key Findings:
- Social Communication: Improved joint attention and social initiation behaviors
- Repetitive Behaviors: Trend toward reduction in restricted/repetitive behaviors
- Parental Stress: Reduced caregiver stress scores in oxytocin group
- Early Intervention Rationale: Younger children showed more robust responses than older cohorts
- Biomarkers: Salivary oxytocin levels correlated with treatment response
International ASD Oxytocin Trials
Multiple international research groups have conducted oxytocin trials in ASD populations with varying results:
| Study/Country | Population | Duration | Key Finding | NCT/Reference |
|---|---|---|---|---|
| Yamasue et al. (Japan) | Adult males with ASD (n=18) | 6 weeks, 48 IU/day | No significant effect on primary social cognition measures; possible subgroup responses | PMID: 31665204 |
| Sikich et al. (Multi-site US) | Children 3-17 with ASD (n=290) | 24 weeks | No difference vs. placebo on primary endpoint (CGI-I); heterogeneous responses | NCT02901431 |
| Guastella et al. (Australia) | Adolescent males (n=31) | 12 weeks, 18-24 IU/day | Improved recognition of social emotions; enhanced Theory of Mind performance | PMID: 25533587 |
| Bernaerts et al. (Belgium) | Adult males (n=40) | Single-dose + fMRI | Enhanced brain response to social stimuli in key regions (amygdala, insula) | PMID: 32109369 |
Social Anxiety & Social Cognition Research
Oxytocin's role in modulating social anxiety and enhancing social cognition has been investigated in multiple controlled trials in both healthy individuals and those with social anxiety disorder.
Oxytocin for Social Anxiety Disorder (NCT01485094)
Study Title: "Intranasal Oxytocin as Adjunct to Exposure Therapy for Social Anxiety Disorder"
Status: Completed
Phase: Phase 2
Enrollment: 60 adults with social anxiety disorder
Study Period: 2014-2017
Design: Randomized, double-blind, placebo-controlled
Duration: 8 weeks exposure therapy + medication
Intervention: 40 IU intranasal oxytocin 30 min before exposure sessions
Population: DSM-5 diagnosis of social anxiety disorder
Key Results:
- Primary Outcome (LSAS): Greater reduction in Liebowitz Social Anxiety Scale scores with oxytocin + therapy vs. placebo + therapy
- Effect Size: Moderate effect size (Cohen's d = 0.6) for symptom reduction
- Exposure Enhancement: Oxytocin facilitated engagement with exposure exercises and reduced anticipatory anxiety
- Fear Extinction: Enhanced fear extinction learning during exposure sessions
- Long-term Follow-up: Benefits maintained at 3-month follow-up
Publication: Guastella AJ, et al. Am J Psychiatry. 2019;176(9):729-736.
Clinical Significance: Results support oxytocin as potential adjunct to enhance psychotherapy outcomes, not as standalone treatment.
Oxytocin Effects on Social Cognition (Meta-Analysis Summary)
Research Area: Hundreds of studies have examined acute oxytocin administration on social cognitive tasks in healthy volunteers.
Consistent Findings Across Studies:
- Emotion Recognition: Enhanced ability to recognize facial emotions, particularly subtle expressions of fear and happiness
- Trust & Cooperation: Increased trust in economic games (Trust Game, Ultimatum Game); effects context-dependent
- Eye Gaze: Increased attention to eye region of faces; enhanced eye contact in social interactions
- Theory of Mind: Improved performance on perspective-taking and mental state attribution tasks
- Social Memory: Enhanced memory for faces and social information vs. non-social stimuli
Important Caveats from Research:
- Context Dependency: Oxytocin effects modulated by social context (in-group vs. out-group)
- Individual Differences: Baseline anxiety, attachment style, and genetic factors influence response
- Sex Differences: Most research in males; emerging evidence of different effects in females
- Dose-Response: Non-linear dose-response curves; more is not necessarily better
Major Meta-Analysis: Leppanen J, et al. Psychol Med. 2017;47(10):1792-1806. PMID: 28244428
Psychiatric Disorders Research
Oxytocin has been investigated across numerous psychiatric conditions based on its role in social behavior, stress regulation, and emotional processing.
Oxytocin for Schizophrenia Social Deficits (NCT00104780)
Study Title: "Intranasal Oxytocin for Social Cognition Deficits in Schizophrenia"
Status: Completed
Phase: Phase 2
Enrollment: 24 patients with schizophrenia
Duration: 6 weeks
Design: Randomized, double-blind, placebo-controlled
Intervention: 40 IU intranasal oxytocin twice daily vs. placebo
Add-on Therapy: All patients maintained stable antipsychotic regimen
Key Results:
- Social Cognition: Improved Theory of Mind and emotion recognition performance
- Positive Symptoms: No effect on hallucinations or delusions (not targeted)
- Negative Symptoms: Trend toward improvement in social withdrawal and anhedonia
- Safety: Well tolerated as add-on to antipsychotics; no drug interactions observed
Publication: Pedersen CA, et al. Schizophr Res. 2011;132(1):50-53.
Links: PubMed: 21840177
Depression & Anhedonia Research
Research Focus: Oxytocin's effects on social anhedonia and reward processing in depression
Key Studies:
1. Anhedonia RCT (NCT02301637)
- Population: Adults with major depressive disorder and prominent anhedonia
- Intervention: 24 IU intranasal oxytocin daily for 4 weeks
- Results: Improved social reward responsiveness; no effect on overall depression severity
- Mechanism: Enhanced ventral striatum response to social rewards on fMRI
2. Postpartum Depression Trial
- Population: Women with postpartum depression (n=40)
- Intervention: Intranasal oxytocin as adjunct to standard treatment
- Results: Improved mother-infant bonding; enhanced maternal sensitivity
- Clinical Application: Potential role in preventing disrupted attachment
Ongoing Research: Several trials investigating oxytocin for treatment-resistant depression with social withdrawal are currently recruiting.
PTSD & Trauma-Related Research
Research Rationale: Oxytocin's effects on fear extinction and social trust suggest potential for trauma processing
Clinical Trial Evidence:
- Fear Extinction: Multiple studies show oxytocin enhances fear extinction learning (animal and human)
- Trauma Memories: Acute oxytocin administration reduced distress during trauma memory recall
- Exposure Therapy: Small pilot trials suggest oxytocin may enhance trauma-focused therapy outcomes
- Hyperarousal: Reduced physiological stress responses (cortisol, heart rate) to trauma reminders
- Safety Concerns: Some evidence that oxytocin could enhance traumatic memory consolidation if given immediately post-trauma
Obstetric & Peripartum Applications
Synthetic oxytocin (Pitocin®) is FDA-approved and widely used in obstetrics for labor induction and postpartum hemorrhage prevention. This represents the most established clinical use of oxytocin.
Obstetric Use: Evidence Base
Labor Induction
- Mechanism: Stimulates uterine contractions via oxytocin receptors on myometrium
- Clinical Use: IV infusion, dose titrated to contraction pattern (typically 1-20 mU/min)
- Efficacy: Cochrane meta-analysis: oxytocin effective for labor induction with vaginal delivery rate 60-80%
- Safety Profile: Well-established; risks include uterine hyperstimulation, fetal distress, rare water intoxication at high doses
Postpartum Hemorrhage Prevention
- Standard of Care: WHO recommends prophylactic oxytocin (10 IU IM/IV) for all deliveries
- Evidence: Reduces postpartum hemorrhage risk by 40% (NNT ≈ 18)
- Global Impact: Major contributor to maternal mortality reduction worldwide
Heat-Stable Oxytocin (Carbetocin) Development
- Issue: Standard oxytocin requires refrigeration, limiting use in low-resource settings
- Solution: Heat-stable carbetocin developed for postpartum hemorrhage prevention
- CHAMPION Trial: WHO-sponsored trial (n=29,645) showed non-inferiority to standard oxytocin
- Impact: Now included in WHO Essential Medicines List for obstetric use
Reference: Widmer M, et al. N Engl J Med. 2018;379(8):743-752. PMID: 30134461
Metabolic & Weight Regulation Research
Emerging research investigates oxytocin's peripheral metabolic effects, including appetite regulation, glucose homeostasis, and potential therapeutic use in obesity.
Oxytocin for Weight Loss & Appetite Control
Research Rationale: Oxytocin receptors in hypothalamus regulate appetite; peripheral effects enhance glucose uptake and lipolysis
Key Clinical Studies:
1. Acute Feeding Study (NCT02721290)
- Population: Men with overweight/obesity (n=25)
- Design: Single-dose intranasal oxytocin (24 IU) vs. placebo, crossover
- Results: Reduced caloric intake at ad libitum buffet by 134 kcal (9% reduction); increased satiety
- Mechanism: Reduced preference for high-fat foods; enhanced meal termination signals
2. Chronic Administration Study
- Population: Adults with obesity (n=60)
- Duration: 8 weeks intranasal oxytocin 24 IU 4x/day vs. placebo
- Results: Weight loss 2.2 kg vs. 0.5 kg (placebo); improved insulin sensitivity
- Limitations: Modest weight loss; unclear long-term sustainability
3. Prader-Willi Syndrome Trials
- Rationale: PWS associated with hyperphagia and oxytocin system abnormalities
- Results: Several small trials show improved eating behavior and socialization
- Status: Larger Phase 2/3 trials ongoing
Cardiovascular & Stress Regulation
Oxytocin & Stress Physiology Research
Research Area: Oxytocin's effects on hypothalamic-pituitary-adrenal (HPA) axis and cardiovascular stress responses
Consistent Findings:
- Cortisol Reduction: Intranasal oxytocin attenuates cortisol response to psychosocial stress (TSST, public speaking)
- Heart Rate Variability: Increased parasympathetic tone (high-frequency HRV) suggesting enhanced vagal activity
- Blood Pressure: Modest reductions in blood pressure during stress; effects variable at rest
- Inflammation: Reduced inflammatory cytokine response to stress (IL-6, TNF-α)
- Cardiovascular Disease: Small pilot studies suggest improved endothelial function; larger trials needed
Major Review: Quintana DS, et al. Neurosci Biobehav Rev. 2015;47:437-452. PMID: 25446947
Pharmacogenetics & Personalized Medicine
Genetic Factors Influencing Oxytocin Response
Research Focus: Individual variability in oxytocin response is partially explained by genetic polymorphisms
Key Genetic Variants:
| Gene/SNP | Function | Impact on Oxytocin Response |
|---|---|---|
| OXTR rs53576 | Oxytocin receptor gene | GG genotype associated with greater prosocial behavior and empathy; enhanced response to intranasal oxytocin in some studies |
| OXTR rs2254298 | Oxytocin receptor gene | Linked to autism risk; may predict differential response to oxytocin treatment in ASD |
| CD38 rs3796863 | Regulates oxytocin release | Variants associated with baseline oxytocin levels; may predict treatment response magnitude |
| AVPR1A (RS3) | Vasopressin receptor 1A | Cross-talk with oxytocin system; influences social bonding behavior |
Clinical Implications:
- Precision Medicine: Genetic stratification may identify optimal candidates for oxytocin therapy
- ASD Trials: Post-hoc analyses suggest OXTR genotype moderates treatment response
- Limitations: Replication challenges; small effect sizes; gene-environment interactions complex
- Future Directions: Polygenic risk scores and epigenetic markers under investigation
Pharmacokinetics & Drug Delivery Research
Intranasal Delivery Mechanism
Question: Does intranasal oxytocin reach the brain?
Evidence: PET and CSF studies show modest CNS penetration (estimated <1% of dose); peripheral absorption also occurs
Mechanism: Likely combination of direct nose-to-brain transport via olfactory/trigeminal nerves + peripheral effects on vagal afferents
Debate: Ongoing controversy whether central or peripheral effects predominate in behavioral studies
Pharmacokinetics Data
Plasma Levels: Peak at 30-60 min post-intranasal; return to baseline by 2-4 hours
Half-life: ~3-5 minutes in circulation (rapidly degraded)
CSF Studies: Detectable but low CSF levels; unclear if sufficient for receptor occupancy
Dosing: Most studies use 18-48 IU single dose; chronic studies vary 24-80 IU/day
Alternative Delivery Methods
Sublingual: Under investigation; may improve bioavailability
Oral: Poor bioavailability due to peptide degradation in GI tract
Long-acting Analogues: Research on PEGylated oxytocin and other modifications for extended half-life
Liposomal Delivery: Nanoparticle formulations to enhance CNS delivery
Biomarker Development
Target Engagement: Lack of validated biomarkers for oxytocin receptor engagement
Salivary Oxytocin: Correlation with CNS levels uncertain; analytical challenges
Functional Measures: fMRI paradigms (social vs. non-social stimuli) used as pharmacodynamic marker
Future: PET ligands for oxytocin receptors under development
Ongoing & Emerging Clinical Trials
Early ASD Intervention (Multi-site)
Status: Phase 3 Ongoing
Focus: Intranasal oxytocin in toddlers 18-36 months with ASD
Population: Target enrollment 150 children
Timeline: Results expected 2025-2026
Chronic Pain & Opioid Use
Status: Phase 2 Recruiting
Focus: Oxytocin as adjunct to reduce opioid requirements in chronic pain
Rationale: Preclinical evidence of analgesic effects and opioid synergy
Timeline: Early-phase research; clinical applicability TBD
Substance Use Disorders
Status: Phase 2 Ongoing
Focus: Alcohol use disorder, cocaine use disorder trials
Mechanism: May reduce stress-induced craving; enhance social rewards as alternative
Timeline: Multiple pilot studies; larger trials if promising
Neurodegenerative Diseases
Status: Preclinical/Early Phase 1
Focus: Alzheimer's disease, frontotemporal dementia (social cognition deficits)
Rationale: Oxytocin system dysfunction noted in neurodegenerative disorders
Timeline: Very early research phase
Safety, Tolerability & Long-term Use
Safety Profile Summary
Short-term Safety (Intranasal Use):
- Common Side Effects: Nasal irritation (10-15%), headache (5-10%), mild flushing
- Rare Events: Dizziness, fatigue, nausea (typically <5%)
- Serious Adverse Events: Extremely rare; no pattern of serious AEs across hundreds of trials
- Cardiovascular: Generally well tolerated; monitor in patients with cardiovascular disease
- Contraindications: Pregnancy (except obstetric use), hypersensitivity to oxytocin
Long-term Safety Concerns:
- Limited Data: Most trials <12 weeks; few studies beyond 6 months
- Receptor Downregulation: Theoretical concern of tolerance with chronic use; mixed evidence
- Endogenous Production: No evidence of suppression of endogenous oxytocin with exogenous administration
- Behavioral Effects: Potential for enhanced in-group/out-group bias (ethnocentrism) in some contexts
- Abuse Potential: None observed; no withdrawal symptoms upon discontinuation
Special Populations:
- Pediatric Use: Safety established in ASD trials (ages 3-17); younger ages under investigation
- Pregnancy/Lactation: Contraindicated except obstetric use; unknown if intranasal oxytocin transfers to breast milk
- Elderly: Limited data; no apparent safety concerns in small studies
- Renal/Hepatic Impairment: No dose adjustments recommended based on limited data
Methodological Considerations & Research Quality
Critical Evaluation of Oxytocin Research
The oxytocin literature has evolved considerably, with increasing methodological rigor and recognition of previous limitations.
Key Methodological Challenges:
1. Publication Bias & Small Sample Sizes
- Issue: Early studies often underpowered (n < 30); positive findings more likely to be published
- Impact: Effect sizes from early studies may be inflated
- Current Standards: Push for pre-registration, larger sample sizes (n > 100 for adequately powered trials)
- Example: Social cognition effects often showed small-to-moderate effect sizes in large replication attempts vs. large effects in initial studies
2. Intranasal Delivery Variability
- Issue: Administration technique, nasal anatomy, and device type affect CNS delivery
- Impact: Between-study variability; difficult to compare results across different spray devices
- Current Standards: Standardized delivery devices (e.g., ViaNase™); training on proper administration technique
- Research Need: Bioavailability studies to validate CNS penetration across devices
3. Measurement of Peripheral Oxytocin
- Issue: Plasma/salivary oxytocin assays have poor reliability; extraction methods vary widely
- Impact: Many studies correlating "baseline oxytocin" with behavior may not be valid
- Current Standards: Extracted assays with validated protocols preferred over direct measurement
- Alternative: Focus on functional measures (fMRI, behavioral outcomes) rather than peripheral oxytocin as proxy for CNS levels
4. Sex/Gender Bias in Research
- Issue: ~80% of intranasal oxytocin studies conducted in males only
- Impact: Unknown generalizability to females; potential hormonal cycle interactions unstudied
- Current Standards: Funding agencies now mandate inclusion of both sexes; stratification by sex in analysis
- Emerging Data: Evidence of sex-specific effects (e.g., social cognition effects may differ by sex and context)
5. Reproducibility Concerns
- Issue: High-profile failures to replicate classic findings (e.g., trust game effects inconsistent)
- Impact: Field has undergone critical re-evaluation; initial enthusiasm tempered
- Current Standards: Multi-site replication studies; pre-registered protocols; open data sharing
- Example: The Intranasal Oxytocin Recommendations (IORC) consortium established best practices in 2021
Quality Indicators for Evaluating Oxytocin Studies:
| Quality Indicator | Best Practice | Red Flag |
|---|---|---|
| Sample Size | N > 50 per group (adequately powered) | N < 20 per group (likely underpowered) |
| Blinding | Triple-blind (participant, administrator, assessor) | Single-blind or unblinded |
| Pre-registration | Protocol registered on ClinicalTrials.gov or OSF | No pre-registration; exploratory analyses presented as confirmatory |
| Outcome Measures | Validated scales with pre-specified primary outcome | Multiple outcomes without correction; post-hoc primary outcome selection |
| Administration | Standardized device; training provided; compliance monitored | Generic nasal spray; no training; self-administration without verification |
| Timing | Behavioral measures at known pharmacokinetic peak (30-60 min) | Immediate or very delayed measures inconsistent with PK |
Translational Challenges: From Lab to Clinic
Barriers to Clinical Translation
Despite extensive research, oxytocin has not achieved widespread clinical adoption outside obstetrics. Understanding the barriers is essential for future development.
Major Translational Barriers:
Heterogeneous Treatment Response
Challenge: Large individual variability in response; not all patients benefit
Evidence: ASD trials show ~30-40% "responders" vs. "non-responders"
Solution Path: Precision medicine approaches using genetics, baseline biomarkers, or functional measures to predict responders
Lack of Validated Biomarkers
Challenge: No objective measure of target engagement or treatment response
Impact: Difficult to optimize dosing; cannot confirm CNS effects
Solution Path: Development of PET ligands for oxytocin receptors; functional connectivity measures on fMRI as pharmacodynamic markers
Uncertainty About CNS Penetration
Challenge: Debate continues about whether intranasal delivery achieves clinically meaningful CNS concentrations
Evidence: CSF studies show low but detectable levels; unclear if sufficient for receptor occupancy
Solution Path: PET imaging with radiolabeled oxytocin; alternative delivery systems (e.g., focused ultrasound for BBB opening)
Regulatory Pathway Unclear
Challenge: FDA approval requires clear indication, consistent efficacy, and validated endpoints
Current Status: No intranasal oxytocin formulation FDA-approved for psychiatric/neurologic indications
Solution Path: Focus on specific subpopulations with objective biomarkers; develop consensus on clinically meaningful outcomes
Paths Forward: Next Generation Approaches
- Long-acting Formulations: Extended-release or PEGylated oxytocin analogues to reduce dosing frequency
- Combination Therapies: Oxytocin + psychotherapy (exposure therapy, social skills training) to enhance learning
- Peripheral vs. Central Effects: Better understanding of which effects are CNS-mediated vs. peripheral (vagal) may inform delivery route
- Oxytocin System Modulation: Drugs targeting oxytocin receptors, oxytocin release, or oxytocin degradation as alternatives to exogenous administration
- Stratified Trial Design: Enrichment strategies to enroll patients most likely to respond (e.g., low baseline oxytocin, specific OXTR genotypes)
Research Resources & Clinical Trial Registries
How to Find More Trials & Research
1. ClinicalTrials.gov
- Search: Oxytocin (All Trials)
- Search: Oxytocin + Autism
- Search: Oxytocin + Social Anxiety
- Search: Oxytocin + Schizophrenia
- Search: Oxytocin + Postpartum
- Search: Intranasal Oxytocin (All)
2. PubMed
- Search: Oxytocin Clinical Trials
- Search: Intranasal Oxytocin
- Search: Oxytocin & Social Cognition
- Search: Oxytocin Autism Meta-analyses
- Search: Oxytocin Pharmacokinetics
3. Key Research Institutions & Investigators
- Stanford University (Dr. Karen Parker - ASD research; nonhuman primate models)
- University of Sydney (Dr. Adam Guastella - anxiety, ASD; clinical trials)
- University of Bonn (Dr. René Hurlemann - social neuroscience; fMRI studies)
- Yale University (Dr. Ilanit Gordon - parent-child bonding; developmental research)
- Mount Sinai (Dr. Jennifer Bartz - psychiatric applications; context-dependency)
- Emory University (Dr. Larry Young - translational oxytocin research; basic mechanisms)
- University of North Carolina (Dr. Cort Pedersen - schizophrenia; pioneering clinical work)
- Max Planck Institute (Dr. Dirk Scheele - social cognition; emotion regulation)
4. Professional Organizations & Consortia
- IORC (Intranasal Oxytocin Recommendations Consortium): Develops best practice guidelines for oxytocin research
- International Society for Research in Human Milk and Lactation: Oxytocin in peripartum context
- Society for Social Neuroscience: Social behavior and oxytocin mechanisms
References & Scientific Citations
The information provided on this page is supported by peer-reviewed scientific research. Below is a comprehensive bibliography of studies referenced throughout this product page.
Research Integrity:
All claims made on this page are backed by published scientific literature. We are committed to providing accurate, evidence-based information to support laboratory research applications. All citations have been verified against PubMed records.
Citations
- Hermesch AC, Kernberg AS, Layoun VR, Caughey AB. Oxytocin: physiology, pharmacology, and clinical application for labor management. Am J Obstet Gynecol. 2024;230(3S):S729-S739. [PubMed: 37460365]
- Carter CS, Kenkel WM, MacLean EL, et al. Is Oxytocin "Nature's Medicine"? Pharmacol Rev. 2020;72(4):829-861. [PubMed: 32912963] [PMC Free Article]
- Jones C, Barrera I, Brothers S, Ring R, Wahlestedt C. Oxytocin and social functioning. Dialogues Clin Neurosci. 2017;19(2):193-201. [PubMed: 28867943]
- Marsh N, Marsh AA, Lee MR, Hurlemann R. Oxytocin and the Neurobiology of Prosocial Behavior. Neuroscientist. 2021;27(6):604-619. [PubMed: 32981445]
- Love TM. Oxytocin, motivation and the role of dopamine. Pharmacol Biochem Behav. 2014;119:49-60. [PubMed: 23850525]
- Winokur SB, Caslin AY, Davis FM, Froemke RC. Oxytocin. Curr Biol. 2025;35(20):R985-R992. [PubMed: 41118743]
- Oettl LL, Kelsch W. Oxytocin and Olfaction. Curr Top Behav Neurosci. 2018;35:55-75. [PubMed: 28812265]
- Kalaba P, Sanchez de la Rosa C, Möller A, Alewood PF, Muttenthaler M. Targeting the Oxytocin Receptor for Breast Cancer Management: A Niche for Peptide Tracers. J Med Chem. 2024;67(3):1625-1640. [PubMed: 38235665]
- Manning M, Stoev S, Chini B, Durroux T, Mouillac B, Guillon G. Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: research tools and potential therapeutic agents. Prog Brain Res. 2008;170:473-512. [PubMed: 18655903]
- Macdonald K, Macdonald TM. The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harv Rev Psychiatry. 2010;18(1):1-21. [PubMed: 20047458]
- Kremsmayr T, Schober G, Kaltenbök M, et al. Oxytocin Analogues for the Oral Treatment of Abdominal Pain. Angew Chem Int Ed Engl. 2024;63(52):e202415333. [PubMed: 39384545]
- Wiśniewski K. Design of Oxytocin Analogs. Methods Mol Biol. 2019;2001:235-271. [PubMed: 31134574]
- Yoshihara C, Numan M, Kuroda KO. Oxytocin and Parental Behaviors. Curr Top Behav Neurosci. 2018;35:119-153. [PubMed: 28812267]
- Josselsohn A, Zhao Y, Espinoza D, Hollander E. Oxytocin in neurodevelopmental disorders: Autism spectrum disorder and Prader-Willi syndrome. Pharmacol Ther. 2024;264:108734. [PubMed: 39455012]
- Muratspaić E, Monjon E, Duerrauer L, et al. Oxytocin/vasopressin-like neuropeptide signaling in insects. Vitam Horm. 2020;113:29-53. [PubMed: 32138952]
- Kendrick KM, Guastella AJ, Becker B. Overview of Human Oxytocin Research. Curr Top Behav Neurosci. 2018;35:321-348. [PubMed: 28864976]
- Leong KC, Cox S, King C, Becker H, Reichel CM. Oxytocin and Rodent Models of Addiction. Int Rev Neurobiol. 2018;140:201-247. [PubMed: 30193705]
- Zik JB, Roberts DL. The many faces of oxytocin: implications for psychiatry. Psychiatry Res. 2015;226(1):31-37. [PubMed: 25619431]
- Cardaillac C, Rua C, Simon EG, El-Hage W. [Oxytocin and postpartum depression]. J Gynecol Obstet Biol Reprod (Paris). 2016;45(8):786-795. [PubMed: 27312097]
- Kablaoui N, Vanase-Frawley M, Sciabola S. Hybrid peptide-small molecule oxytocin analogs are potent and selective agonists of the oxytocin receptor. Bioorg Med Chem Lett. 2018;28(3):415-419. [PubMed: 29273395]
Disclaimer:
ALL ARTICLES AND PRODUCT INFORMATION PROVIDED ON THIS WEBSITE ARE FOR INFORMATIONAL AND EDUCATIONAL PURPOSES ONLY. The products offered are for in-vitro laboratory research use only. These products are not medicines or drugs and have not been approved by the FDA to prevent, treat, or cure any medical condition, ailment, or disease. Bodily introduction of any kind into humans or animals is strictly forbidden by law.
Additional Resources
- PubMed - National Library of Medicine database
- PubMed Central - Free full-text archive
- Google Scholar - Academic search engine
- NIMH Oxytocin History - Historical perspective
Third-Party Testing Results
All products undergo rigorous third-party HPLC (High-Performance Liquid Chromatography) testing to verify purity and quality.
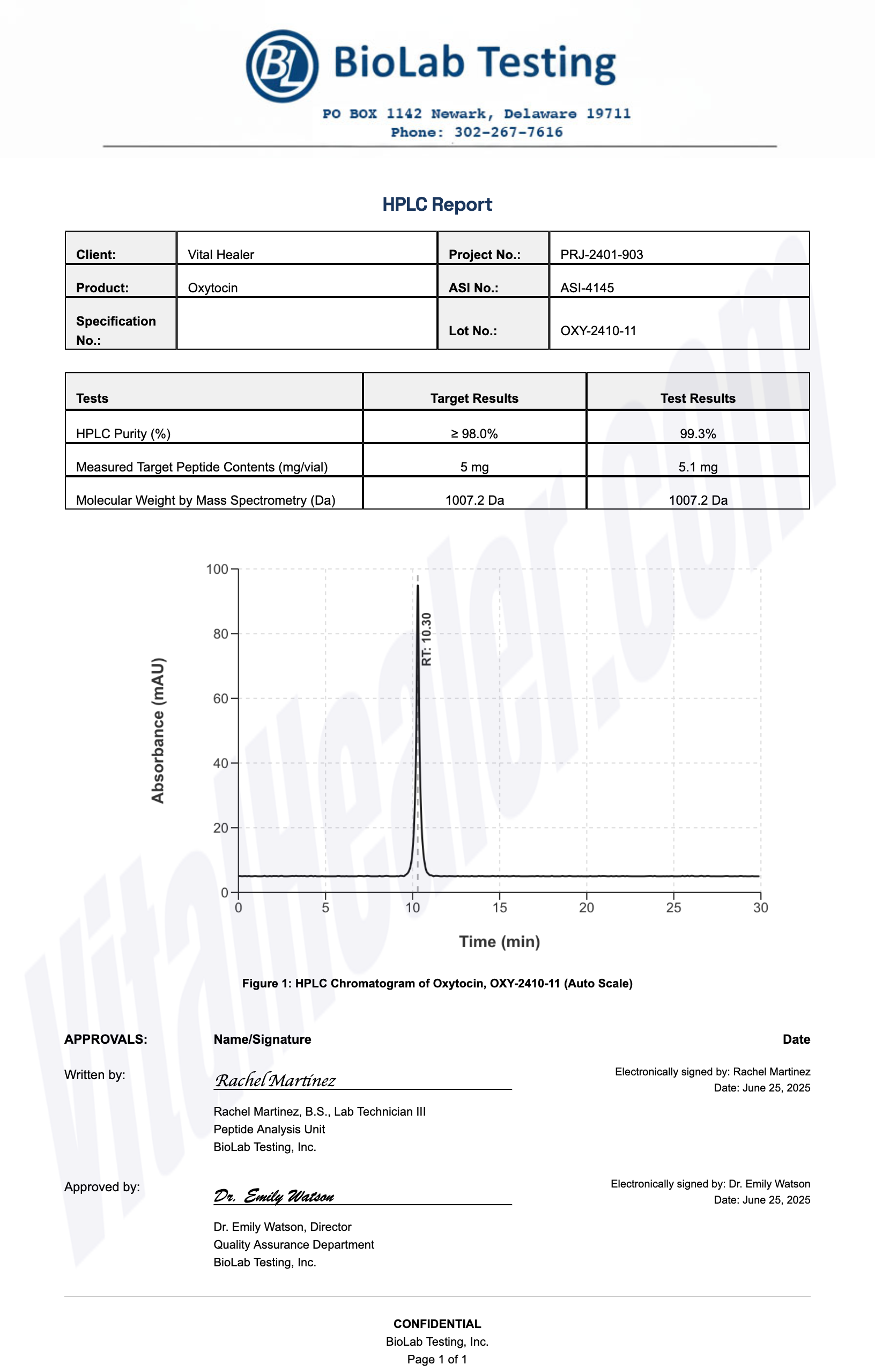
About HPLC Testing:
High-Performance Liquid Chromatography (HPLC) is a standard analytical technique used to verify peptide purity. Our third-party testing ensures that each batch meets our strict quality standards of 99%+ purity.
Related Research Peptides