Kisspeptin-10 (KP-10)
Kisspeptin-10, a critical RF-amide peptide that activates the hypothalamic-pituitary-gonadal axis and regulates reproductive function.
Key Research Properties:
| SKU: | kisspeptin-10 |
|---|---|
| Purity: | >99% (HPLC Verified) |
| Form: | Lyophilized Powder |
| Storage: | Store at -20°C |
| CAS Number: | 885084-21-9 |
| Lot Number: | KSS-2410-04: 5mg |
All products are sold strictly for laboratory and research purposes. Products are not intended for human use or consumption of any kind.
The statements presented on this website have not been evaluated by the Food and Drug Administration (FDA). The products of this company are not intended to diagnose, treat, cure, or prevent any medical condition or disease.
What is Kisspeptin-10 (KP-10)?
Kisspeptin-10 (KP-10) is the decapeptide fragment of the KISS1 gene product that binds the G-protein coupled receptor KISS1R/GPR54 on hypothalamic GnRH neurons, rapidly stimulating pulsatile gonadotropin release and coordinating hypothalamic-pituitary-gonadal (HPG) axis function[1]-[4].
Biochemical Features
- Sequence: Tyr-Asn-Trp-Asn-Ser-Phe-Gly-Leu-Arg-Phe-NH2 (metastin 45–54)
- Mode of Action: High-affinity agonist of KISS1R triggering IP3/Ca2+ signaling and GnRH release[1], [3]
- Physiology: Essential regulator of puberty onset, reproductive competence, and luteinizing hormone (LH)/follicle stimulating hormone (FSH) dynamics[2], [3]
- Pharmacokinetics: Rapid plasma clearance (~15–20 minutes) necessitating bolus or pulsatile infusion paradigms in clinical research[3], [4], [16]
Primary Research Applications
- HPG Axis Diagnostics: Evaluate GnRH neuron integrity in congenital or functional hypogonadotropic hypogonadism[4], [16], [17]
- Women’s Health: Investigate menstrual recovery, hyperprolactinemia, and ovarian stimulation paradigms[8], [9], [15]
- Peripheral Biology: Examine placental invasion, vascular remodeling, bone formation, and cardiometabolic responses to KISS1R activation[6], [7], [10]-[12]
Human infusion studies consistently show that KP-10 produces rapid surges in LH (and downstream sex steroids) within minutes of dosing, validating its use as a dynamic test of GnRH neuron function in both healthy volunteers and patients with reproductive hormone deficiencies[2], [3], [4], [16].
Discovery & Development Milestones
- 2003–2004: Central and peripheral KP-10 administration shown to activate the HPG axis in rodents and humans[1].
- 2011: First-in-man bolus studies demonstrated dose-dependent LH release with sex-specific responses to sustained infusion[2], [3].
- 2015: Comparative trials clarified differential potency and desensitisation between KP-10, KP-54, and GnRH[4].
- 2020s: Pulsatile and subcutaneous delivery paradigms are being explored for chronic therapy in hypogonadism, PCOS, and hypothalamic amenorrhea[16]-[20].
Molecular & Chemical Information
Chemical Structure
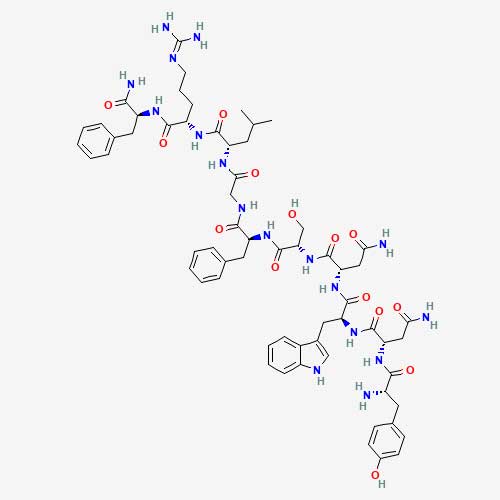
KP-10 is an amidated decapeptide; the C-terminal Arg-Phe-NH2 motif is required for high-affinity KISS1R engagement[5].
| Property | Specification |
|---|---|
| CAS Number | 885084-21-9 |
| Molecular Formula | C63H83N17O14 |
| Molecular Weight | ≈1302.5 g/mol |
| Synonyms | KP-10, metastin 45–54, KISS1 (112–121) |
| Receptor Target | KISS1R/GPR54 (class A GPCR) |
| Structure-Activity Notes | Truncation to the RFamide core abrogates signaling; analogues with enhanced stability are under development for therapeutic use[5]. |
Mechanism of Action
KP-10 is a high-affinity agonist of the KISS1R/GPR54 receptor. Engagement of KISS1R activates phospholipase C, elevates intracellular Ca2+, and provokes GnRH release from hypothalamic neurons, rapidly translating into pituitary LH/FSH secretion and downstream gonadal steroidogenesis[1]-[4].
Hypothalamic GnRH Activation
KISS1R Signalling in the Hypothalamus
- Receptor Density: KISS1R is concentrated on GnRH neurons in the arcuate and preoptic nuclei, positioning KP-10 to directly ignite the GnRH pulse generator[1], [3].
- Second Messenger Cascade: PLC/IP3/Ca2+ signalling promotes vesicular GnRH exocytosis within minutes of KP-10 exposure[1], [3].
- Feedback Integration: Sex steroids modulate KISS1 neuron activity, explaining the sex-specific responsiveness seen with continuous infusion paradigms[2], [4].
- Tachyphylaxis: Sustained exposure can desensitise KISS1R signalling, prompting current trials to test pulsatile or intermittent dosing strategies[4], [18].
Pituitary & Gonadal Responses
Dynamic Endocrine Output
- Rapid Gonadotropin Surges: Intravenous bolus dosing elicits LH (and secondary FSH) increases within 10–15 minutes in healthy men and women[2], [3].
- Sex-Specific Dynamics: Continuous infusion produces greater LH responses in women, reflecting sex steroid feedback on hypothalamic KISS1 neurons[2].
- Restoring Pulsatility: KP-10 can reactivate suppressed gonadotropin secretion in hyperprolactinemia and hypothalamic amenorrhea, supporting its diagnostic value[9], [15].
- Controlled Suppression: Prolonged low-dose infusion is being evaluated to dampen the reproductive axis therapeutically in PCOS and other conditions[18].
Peripheral KISS1R Biology
Beyond the HPG Axis
- Placental Function: KP-10 restrains trophoblast invasion and modulates placental angiogenesis—mechanisms implicated in preeclampsia risk[6], [7], [10].
- Ovarian Hyperstimulation: KP-10 attenuates VEGF-driven vascular leakage in experimental OHSS models, highlighting a potential protective role in fertility treatments[8].
- Bone & Metabolism: KISS1R activation promotes osteoblast differentiation and remodels myocardial collagen, underscoring broader cardiometabolic effects[11], [12].
- Oncology: KP-10 suppresses angiogenesis and metastatic signalling in breast and endometrial cancer models via KISS1R pathways[10].
Research & Evidence
Modern KP-10 research centres on controlled human infusion studies, translational endocrine trials, and peripheral biology investigations that map KISS1R signalling across reproductive and somatic tissues[2]-[12], [16]-[18].
Hypogonadotropic Hypogonadism & Amenorrhea
Restoring GnRH-Driven Pulsatility
- Congenital IHH: Pulsatile intravenous KP-10 reactivates LH secretion in individuals with congenital GnRH deficiency, demonstrating intact pituitary responsiveness[3], [16], [18].
- Functional Hypothalamic Disorders: Subcutaneous protocols under investigation aim to normalise gonadotropins in hypothalamic amenorrhea and related functional disorders[17].
- Hyperprolactinemia: KP-10 infusion reactivates LH/FSH pulsatility in women with chronic hyperprolactinemia and amenorrhea, offering a dynamic diagnostic tool[9], [15].
PCOS, Hyperprolactinemia & Reproductive Endocrinology
Women’s Health Applications
- PCOS Modulation: Continuous low-dose KP-10 is being studied to dampen LH hypersecretion and androgen excess in PCOS[18].
- Hyperprolactinemic Infertility: Acute KP-10 boluses can bypass prolactin-mediated GnRH suppression, transiently restoring gonadotropin output[9], [15].
- Comparative Pharmacology: Direct comparisons with KP-54 and GnRH provide dosing benchmarks for future therapeutic analogues[4], [5].
Pregnancy & Placental Biology
Maternal-Fetal Insights
- Preeclampsia Biomarker: Maternal KP-10 concentrations correlate with fetal wellbeing metrics in preeclamptic pregnancies[6].
- Trophoblast Invasion: KP-10 restrains excessive trophoblast migration, supporting controlled placental implantation[7].
- Placental Angiogenesis: KP-10 limits endothelial sprouting and VEGF signalling, with implications for OHSS prevention and placental vascular homeostasis[8], [10].
Peripheral & Oncologic Research
Emerging KISS1R Targets
- Anti-Metastatic Potential: KP-10 suppresses invasion and chemotactic signalling in breast and endometrial cancer cell models[10].
- Bone Formation: KP-10 promotes osteoblast differentiation and BMP2 expression, suggesting skeletal benefits[11].
- Cardiovascular Remodeling: Chronic KP-10 exposure increases myocardial collagen content via focal adhesion kinase signalling, warranting cardiovascular safety monitoring[12].
Dosing & Administration
Controlled Human Protocols
Intravenous Bolus & Short Infusion
- Bolus Testing: Low nanomole-per-kilogram intravenous boluses provoke rapid LH surges in healthy volunteers and patients, enabling dynamic assessment of GnRH neuron integrity[2], [3].
- Short Infusions: 60–180 minute infusions characterise sex-specific feedback and help delineate tachyphylaxis thresholds during sustained exposure[2], [4].
- Sampling: Frequent serum LH/FSH sampling (5–10 minute intervals) is required to resolve pulsatile responses[2], [3].
Pulsatile & Continuous Delivery
Emerging Infusion Strategies
- Pulsatile Pumps: Clinical trials are testing programmable intravenous pumps delivering KP-10 every 60–120 minutes to mimic endogenous GnRH pulsatility in hypogonadal patients[16], [18].
- Subcutaneous Infusion: Phase 1/2 studies are exploring subcutaneous delivery for chronic management of hypothalamic amenorrhea and PCOS while monitoring for desensitisation[17], [18].
- Combination Protocols: KP-10 is being combined with dopamine agonists or gonadotropins to assess synergistic restoration of fertility parameters[9], [15].
Preparation & Handling
- Reconstitution: Dissolve lyophilised KP-10 in sterile saline or bacteriostatic water immediately prior to use; filter sterilisation is recommended for infusion preparations.
- Storage: Maintain lyophilised material at -20 °C; keep reconstituted solutions refrigerated (2–8 °C) and discard after the validated stability window for the specific study protocol.
- Delivery Apparatus: Use low-binding infusion sets and minimise dwell time to counter rapid adsorption and degradation typical of short RFamide peptides[5].
- Monitoring: Continuous cardiovascular and endocrine monitoring is advised during human infusions due to the peptide’s potent endocrine activity.
Safety & Side Effects
Supervised infusion studies show largely mild adverse events, but chronic KP-10 exposure requires vigilance for desensitisation and peripheral effects[2]-[4], [16]-[18], [12].
Acute Tolerability
- Transient Reactions: Flushing, heat sensation, mild headache, and occasional nausea resolve rapidly after dosing[2], [3].
- Cardiovascular Profile: Acute bolus and short infusions have not produced clinically meaningful blood-pressure or heart-rate changes in controlled cohorts[2], [3].
- Hormonal Overshoot: LH/FSH spikes subside quickly once infusion ends, but serial sampling is recommended to detect exaggerated responses[3], [4].
Chronic & Pulsatile Delivery
- Desensitisation Risk: Continuous exposure can blunt gonadotropin responses, guiding current trials toward pulsatile pumps and scheduled breaks[4], [16], [18].
- Local Reactions: Subcutaneous studies occasionally report injection-site erythema or tenderness that resolves with site rotation[17], [18].
- Intentional Suppression: Protocols aimed at dampening LH (e.g., PCOS) require monitoring to prevent hypoestrogenic symptoms and unintended infertility[18].
Peripheral Considerations
- Placental & Vascular Effects: KP-10 regulates trophoblast invasion and angiogenesis; pregnancy-related research must incorporate maternal-fetal surveillance[6], [10].
- Cardiometabolic Signals: Chronic exposure increases myocardial collagen content in animal models, highlighting a potential cardiovascular liability[12].
- Oncology & Bone: Anti-metastatic and osteogenic actions warrant tissue-specific toxicology to balance efficacy and safety[10], [11].
Frequently Asked Questions
Clinical Trials & Research Status
Academic centres in Boston, London, and beyond are running phase 1/2 studies to characterise KP-10 for hypogonadism, PCOS, and hypothalamic amenorrhea, while earlier proof-of-concept trials have clarified dosing and endocrine responses[13]-[20].
Completed Interventional Trials
NCT04648969 • Prolonged Pulsatile Kisspeptin in Hypogonadotropic Hypogonadism
- Status: Completed (Massachusetts General Hospital, USA)[16]
- Design: Interventional, single-arm; programmable IV pump delivering pulsatile KP-10 for several days.
- Participants: Adults with congenital or acquired hypogonadotropic hypogonadism.
- Primary Endpoints: Restoration of LH pulsatility and sex-steroid production; safety/tolerability assessments.
- ClinicalTrials.gov: NCT04648969
NCT02956447 • Kisspeptin Administration in Hyperprolactinemia (WITH RESULTS)
- Status: Completed with posted results (Massachusetts General Hospital, USA)[15]
- Design: Randomised crossover; IV KP-10 vs. vehicle during frequent blood sampling.
- Population: Women with hyperprolactinemia-induced hypogonadotropic hypogonadism.
- Primary Outcomes: Change in LH pulse frequency/amplitude; secondary endocrine and safety metrics.
- ClinicalTrials.gov: NCT02956447
NCT05971849 • Dampening the Reproductive Axis With Continuous Kisspeptin
- Status: Completed (Massachusetts General Hospital, USA)[18]
- Design: Interventional, single-arm continuous infusion to explore receptor desensitisation.
- Indication: Polycystic ovary syndrome and related reproductive disorders requiring LH suppression.
- Endpoints: LH/FSH suppression kinetics, metabolic biomarkers, infusion tolerability.
- ClinicalTrials.gov: NCT05971849
NCT01438073 • Elucidating Kisspeptin Physiology by Blocking Kisspeptin Signaling
- Status: Completed (Brigham and Women’s Hospital, USA)[13]
- Design: Randomised, double-blind antagonist study assessing endogenous kisspeptin tone.
- Population: Healthy postmenopausal women, hypogonadotropic men, and women with hypothalamic dysfunction.
- Primary Outcomes: Changes in LH, FSH, sex steroids, and metabolic hormones following kisspeptin blockade.
- ClinicalTrials.gov: NCT01438073
Active & Recruiting Studies
NCT02081924 • Reproductive Hormones During Sustained Kisspeptin Administration
- Status: Recruiting (Imperial College London, UK)[14]
- Design: Stepwise dose-escalation infusions to profile chronic endocrine responses.
- Participants: Adults with fertility disorders or hypothalamic dysfunction.
- Primary Endpoints: LH/FSH secretion dynamics, sex steroid levels, safety laboratory values.
- ClinicalTrials.gov: NCT02081924
NCT07224438 • Subcutaneous Kisspeptin for Hypothalamic Amenorrhea
- Status: Recruiting (Massachusetts General Hospital, USA)[17]
- Design: Outpatient subcutaneous infusion or bolus protocols with menstrual cycle tracking.
- Population: Women diagnosed with functional hypothalamic amenorrhea.
- Primary Outcomes: LH pulsatility restoration, ovulatory markers, safety/tolerability.
- ClinicalTrials.gov: NCT07224438
NCT05896293 • Subcutaneous Kisspeptin in Idiopathic Hypogonadotropic Hypogonadism (IHH)
- Status: Recruiting (Massachusetts General Hospital, USA)[19]
- Design: Single-group, repeated subcutaneous administrations with endocrine monitoring.
- Participants: Adults with congenital or idiopathic hypogonadotropic hypogonadism.
- Primary Outcomes: LH/FSH secretion, sex steroid responses, safety labs, anti-drug antibodies.
- ClinicalTrials.gov: NCT05896293
NCT05633966 • Subcutaneous Kisspeptin in Reproductive Disorders
- Status: Completed; follow-up analyses ongoing (Massachusetts General Hospital, USA)[18]
- Design: Multiple-dose subcutaneous administration across several reproductive phenotypes.
- Objectives: Safety, endocrine pharmacodynamics, feasibility of home-based infusion systems.
- ClinicalTrials.gov: NCT05633966
References & Scientific Citations
Research Integrity:
References include landmark Kisspeptin-10 studies from peer-reviewed journals.
- Thompson EL, Patterson M, Murphy KG, et al. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic–pituitary–gonadal axis. J Neuroendocrinol. 2004;16(10):850-858. doi:10.1111/j.1365-2826.2004.01240.x
- Jayasena CN, Nijher GMK, Abbara A, et al. The effects of kisspeptin-10 on reproductive hormone release show sexual dimorphism in humans. J Clin Endocrinol Metab. 2011;96(12):E1963-E1972. doi:10.1210/jc.2011-1454
- George JT, Veldhuis JD, Roseweir AK, et al. Kisspeptin-10 is a potent stimulator of LH and increases pulse frequency in men. J Clin Endocrinol Metab. 2011;96(8):E1228-E1236. doi:10.1210/jc.2011-0087
- Jayasena CN, Abbara A, Narayanaswamy S, et al. Direct comparison of the effects of intravenous kisspeptin-10, kisspeptin-54 and GnRH on gonadotrophin secretion in healthy men. Hum Reprod. 2015;30(8):1934-1943. doi:10.1093/humrep/dev141
- Curtis AE, Cooke JH, Baxter JE, et al. A kisspeptin-10 analog with greater in vivo bioactivity than kisspeptin-10. Am J Physiol Endocrinol Metab. 2010;298(2):E296-E303. doi:10.1152/ajpendo.00426.2009
- Ziyaraa MA, Hamdan FB, Mousa LR. Correlation of kisspeptin-10 level and fetal well-being in preeclamptic patients. Taiwanese J Obstet Gynecol. 2016;55(3):343-349. doi:10.1016/j.tjog.2016.04.029
- Bilban M, Tauber S, Haslinger P, et al. Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J Cell Sci. 2004;117(8):1319-1328. doi:10.1242/jcs.00990
- Zhai J, Liu J, Zhao S, et al. Kisspeptin-10 inhibits OHSS by suppressing VEGF secretion. Reproduction. 2017;154(4):R135-R143. doi:10.1530/REP-17-0268
- Millar RP, Sonigo C, Anderson RA, et al. Hypothalamic-pituitary-ovarian axis reactivation by kisspeptin-10 in hyperprolactinemic women with chronic amenorrhea. J Endocr Soc. 2017;1(11):1362-1371. doi:10.1210/js.2017-00328
- Ramaesh T, Logie JJ, Roseweir AK, et al. Kisspeptin-10 inhibits angiogenesis in human placental vessels ex vivo and endothelial cells in vitro. Endocrinology. 2010;151(12):5927-5937. doi:10.1210/en.2010-0505
- Son HE, Kim KM, Kim EJ, Jang WG. Kisspeptin-10 stimulates osteoblast differentiation through GPR54-mediated regulation of BMP2 expression and activation. Sci Rep. 2018;8:20571. doi:10.1038/s41598-018-20571-2
- Radwańska P, Gałądzyńska M, Piera L, Drobnik J. Kisspeptin-10 increases collagen content in the myocardium by focal adhesion kinase activity. Sci Rep. 2023;13:15040. doi:10.1038/s41598-023-47224-3
- ClinicalTrials.gov. Elucidating Kisspeptin Physiology by Blocking Kisspeptin Signaling. Identifier: NCT01438073. https://clinicaltrials.gov/study/NCT01438073
- ClinicalTrials.gov. Reproductive Hormones During Sustained Administration of Kisspeptin. Identifier: NCT02081924. https://clinicaltrials.gov/study/NCT02081924
- ClinicalTrials.gov. Administration of Kisspeptin in Patients With Hyperprolactinemia. Identifier: NCT02956447. https://clinicaltrials.gov/study/NCT02956447
- ClinicalTrials.gov. Prolonged Pulsatile Kisspeptin Administration in Hypogonadotropic Hypogonadism. Identifier: NCT04648969. https://clinicaltrials.gov/study/NCT04648969
- ClinicalTrials.gov. Kisspeptin Administration Subcutaneously to Patients With Hypothalamic Amenorrhea. Identifier: NCT07224438. https://clinicaltrials.gov/study/NCT07224438
- ClinicalTrials.gov. Kisspeptin Administration Subcutaneously to Patients With Reproductive Disorders. Identifier: NCT05633966. https://clinicaltrials.gov/study/NCT05633966
- ClinicalTrials.gov. Kisspeptin Administration Subcutaneously to Patients With IHH. Identifier: NCT05896293. https://clinicaltrials.gov/study/NCT05896293
- ClinicalTrials.gov. Dampening the Reproductive Axis With Continuous Kisspeptin. Identifier: NCT05971849. https://clinicaltrials.gov/study/NCT05971849
Third-Party Testing Results
All products undergo rigorous third-party HPLC (High-Performance Liquid Chromatography) testing to verify purity and quality.
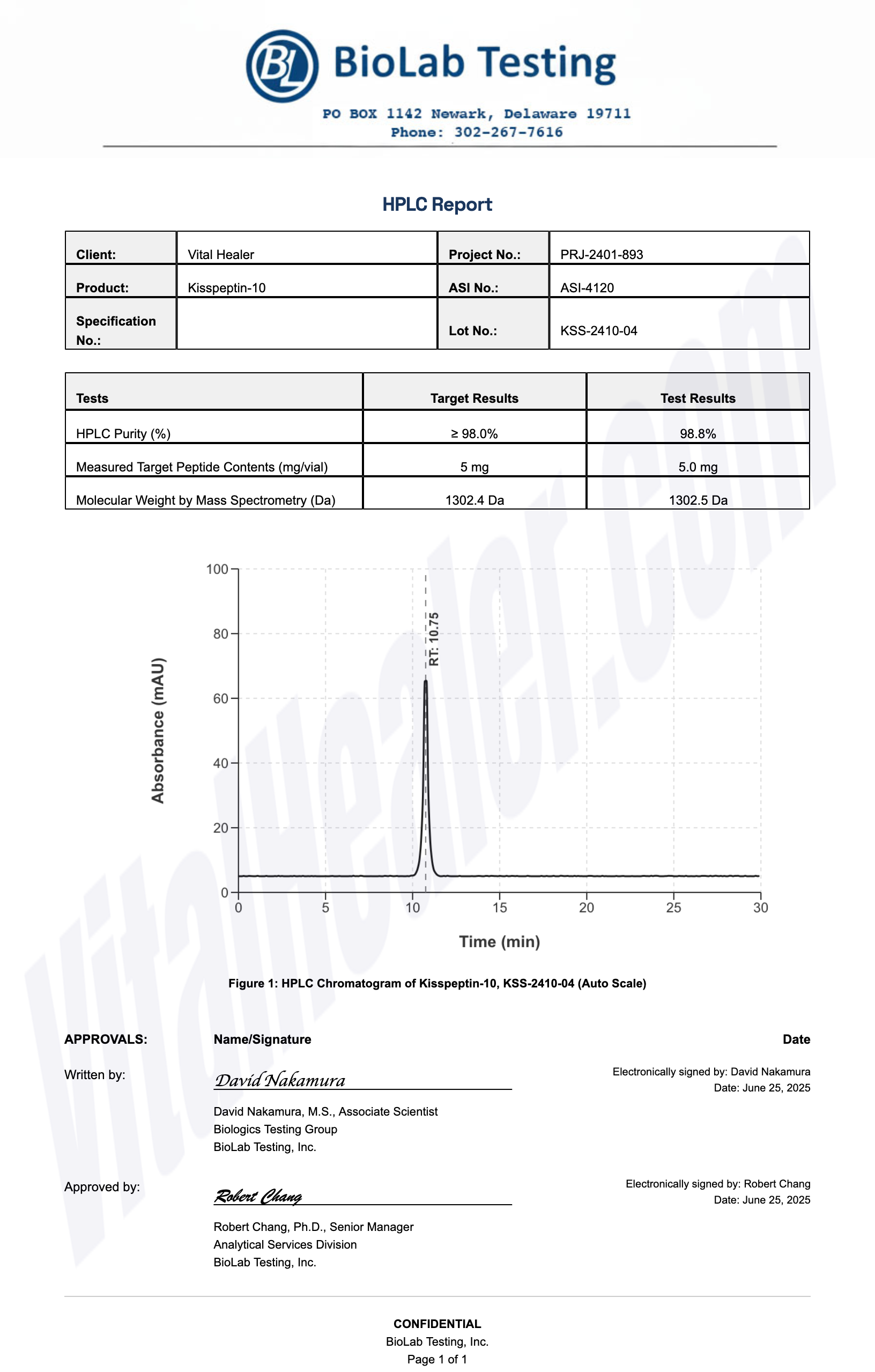
About HPLC Testing:
High-Performance Liquid Chromatography (HPLC) is a standard analytical technique used to verify peptide purity. Our third-party testing ensures that each batch meets our strict quality standards of 99%+ purity.
Related Research Peptides