ll-37
A cytomed‑originated peptide complex often discussed in the context of cellular regulation.
Key Research Properties:
| SKU: | ll-37 |
|---|---|
| Purity: | >99% (HPLC Verified) |
| Form: | Lyophilized Powder |
| Storage: | Store at -20°C |
| CAS Number: | 195875-84-4 |
| Lot Number: | LL3-2410-08: 5mg |
All products are sold strictly for laboratory and research purposes. Products are not intended for human use or consumption of any kind.
The statements presented on this website have not been evaluated by the Food and Drug Administration (FDA). The products of this company are not intended to diagnose, treat, cure, or prevent any medical condition or disease.
What is LL-37?
LL-37 is the sole human cathelicidin peptide, generated when the hCAP18 precursor is proteolytically processed into a 37–amino acid, cationic α-helix that binds microbial membranes and tunes innate immune responses[1], [2]. Because it simultaneously kills pathogens, coordinates chemotaxis, and supports tissue repair, LL-37 has become a central biomarker and experimental therapeutic in mucosal immunity, dermatology, and infectious disease research[3], [5].
Molecular Fingerprint
- Precursor: hCAP18 (
CAMPgene) stored in neutrophil granules and epithelial secretory vesicles[1] - Sequence: LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES with net charge +6 at physiological pH[1]
- Processing: Proteinase 3, kallikreins, and other serine proteases liberate active LL-37 from hCAP18[4]
- Structure: Amphipathic α-helix that dimerises on lipid bilayers and nucleic acids[1], [4]
- Baseline Sources: Neutrophils, airway and gut epithelia, keratinocytes, salivary glands, sweat[2], [3]
Functional Portfolio
- Broad-spectrum antimicrobial: Rapidly perforates bacterial and fungal membranes at micromolar concentrations[1], [6]
- Immune choreography: Engages FPR2, P2X7, and EGFR to drive chemotaxis, cytokine tuning, and barrier repair[2], [4]
- Wound and angiogenic support: Stimulates keratinocyte migration, fibroblast proliferation, and endothelial sprouting[5]
- Oncology signal: Aberrant LL-37 contributes to tumor immune evasion or suppression in a context-dependent manner[5]
- Antiviral defense: Blocks viral fusion and enhances interferon pathways in respiratory and mucosal infections[7], [10]
Peptide Illustration
LL-37 structural artwork, Vital Healer graphics archive.
| Property | Value |
|---|---|
| CAS Number | 195875-84-4 |
| Length | 37 amino acids (residues 134-170 of hCAP18) |
| Molecular Weight | ≈ 4.49 kDa[1] |
| Net Charge / pI | +6 at neutral pH; isoelectric point ≈ 11.0[1] |
| Precursor Gene | CAMP (chromosome 3p21.31)[2] |
| Primary Processing Enzymes | Neutrophil proteinase 3; epidermal kallikrein 5 and 7[4] |
Vitamin D axis
1,25(OH)2D regulates CAMP transcription via VDRE motifs, linking sunlight and supplementation to LL-37 availability[3].
Mechanism of Action
LL-37 operates through a dual-action model: rapid membrane disruption that eliminates microbes, and receptor-driven signalling that recalibrates innate and adaptive responses[1], [2].
Direct Antimicrobial Killing
- Carpet & toroidal pore formation: The amphipathic helix decorates microbial membranes, collapses membrane potential, and causes leakage at 1–10 µM concentrations[1].
- Biofilm disruption: LL-37 penetrates extracellular polymeric matrices, interferes with quorum sensing, and sensitises biofilm bacteria to antibiotics[6].
- Viral envelope destabilisation: The peptide binds viral glycoproteins and prevents fusion, complementing its ability to enhance interferon responses[10].
Host Signalling & Immune Modulation
- Pattern-recognition tuning: LL-37 neutralises LPS/LTA and suppresses excessive TLR4 signalling while still permitting pathogen clearance[2], [3].
- Receptor activation: Engagement of FPR2, P2X7, EGFR, and IGF1R triggers chemotaxis, cytokine release, autophagy, and epithelial restitution[2], [4].
- Immune cell recruitment: LL-37 gradients recruit neutrophils, monocytes, mast cells, and T cells to sites of injury, linking innate and adaptive immunity[3].
Tissue Repair & Resolution
- Keratinocyte and fibroblast activation: LL-37 transactivates EGFR to accelerate migration and proliferation during re-epithelialisation[5].
- Angiogenesis: It induces VEGF and stimulates endothelial tube formation, improving perfusion in ischemic tissue[5].
- Autoimmune balance: Dysregulated LL-37–DNA complexes can aberrantly trigger plasmacytoid dendritic cells, linking the peptide to lupus and psoriasis pathogenesis[7].
Research & Evidence
Broad-Spectrum Antimicrobial Activity
Planktonic Pathogens
LL-37 disrupts bacterial and fungal membranes within micromolar ranges, producing rapid loss of membrane potential and cell death[8].
- Kills Gram-positive and Gram-negative bacteria, including Pseudomonas and Staphylococcus
- Exerts fungicidal effects against Candida species
- Displays virucidal activity against enveloped viruses
Biofilm Disruption
LL-37 penetrates and destabilises bacterial biofilms, reducing biomass and sensitising communities to antibiotics[9].
- Prevents biofilm establishment on abiotic surfaces
- Down-regulates quorum sensing pathways
- Acts synergistically with conventional antibiotics
Immune Modulation & Vitamin D Axis
Innate Immune Signalling
LL-37 modulates Toll-like receptor signalling, chemotaxis, and cytokine production, acting as a multifunctional host-defence peptide[7], [12].
- Neutralises LPS/LTA to prevent excessive TLR4 activation
- Engages FPR2/P2X7 receptors to guide leukocyte recruitment
- Shapes dendritic cell and macrophage differentiation
Vitamin D-Induced Expression
Active vitamin D (1,25(OH)2D3) directly induces CAMP gene transcription, linking nutritional status to LL-37 output and innate immunity[4].
- Vitamin D receptor binding to CAMP promoter elevates LL-37 mRNA
- Supplementation studies show increased circulating LL-37
- Explains epidemiologic links between vitamin D deficiency and infection risk
Tissue Repair & Barrier Homeostasis
Wound Healing
LL-37 accelerates re-epithelialisation, enhances keratinocyte migration, and promotes angiogenesis in cutaneous wounds[5], [11].
- Topical LL-37 improves closure of chronic ulcers and burn models
- Regulates growth factors (EGFR, VEGF) critical for tissue repair
- Serves as biomarker for non-healing wounds
Barrier & Disease Contexts
Aberrant LL-37 expression contributes to inflammatory skin disorders (rosacea, psoriasis) and displays context-dependent roles in cancer biology[6], [10].
- Excess proteolytic processing drives rosacea inflammation
- LL-37–nucleic acid complexes can activate autoimmune pathways
- Tumour microenvironment dictates pro- vs. anti-tumour LL-37 effects
Dosing & Administration
Effective LL-37 concentrations depend on the experimental model, ranging from micromolar antimicrobial assays to topical formulations evaluated in animal and human studies[8]-[11], [13], [14].
Clinical Trial Dosing (Human Research)
Clinical trial protocols:
- Intratumoral melanoma study (NCT02225366): Weekly injections into 2–4 lesions for eight weeks across escalating dose cohorts[13]
- Diabetic foot ulcer study (NCT04098562): 0.5 mg/mL LL-37 cream applied twice weekly for four weeks alongside standard wound care[14]
Use these published regimens as reference points when designing translational research or formulation studies.
Storage & Handling
Storage Conditions
- Lyophilized powder: -20°C or -80°C
- Reconstituted solution: Store at -20°C; avoid repeated freeze-thaw
- Working aliquots: Store at 4°C for up to 1 week
- Light-sensitive: Store in amber vials or wrapped in foil
Reconstitution
- Solvent: Sterile water, PBS, or acetic acid (0.01% for long-term storage)
- Concentration: Prepare 1 mg/mL stock solution
- Method: Gently dissolve; avoid vigorous vortexing
- Aliquoting: Prepare single-use aliquots to minimize freeze-thaw cycles
Stability Notes
- LL-37 is relatively stable in solution at pH 5-7
- Susceptible to proteolytic degradation in serum
- Add protease inhibitors for cell culture studies with serum
- Verify purity by HPLC/mass spec after prolonged storage
Safety & Side Effects
Published clinical protocols and preclinical studies report favourable safety profiles for LL-37 in controlled topical and intratumoral applications[8], [10], [13], [14].
Clinical Safety Data
Safety Profile from Human Clinical Trials
Registry takeaways:
- Topical LL-37 (NCT04098562): Study design emphasises local tolerability; no LL-37–attributed serious adverse events reported in the registry synopsis[14].
- Intratumoral LL-37 (NCT02225366): Weekly dosing guided by CTCAE monitoring; registry description notes dose-escalation without predefined systemic toxicity signals[13].
- Systemic exposure: Preclinical pharmacokinetic studies suggest minimal systemic uptake following topical delivery, consistent with clinical observations[10].
Preclinical Safety Considerations
Preclinical Observations
Potential Risks & Contraindications
Theoretical Concerns (Limited Clinical Data):
Autoimmune Exacerbation:
LL-37–nucleic acid complexes activate plasmacytoid dendritic cells; model autoimmune settings (psoriasis, lupus) with caution[6].
Cancer Considerations:
LL-37 can either inhibit or support tumour growth depending on microenvironmental cues; incorporate rigorous controls in oncologic studies[10].
Excessive Inflammation:
High concentrations may amplify pro-inflammatory signalling; titrate doses to balance antimicrobial and immunomodulatory effects[7], [12].
Hypersensitivity:
Peptide-based agents can elicit allergic responses in sensitised subjects; monitor for hypersensitivity during exploratory dosing.
Laboratory Safety
Standard Laboratory Precautions:
- Wear appropriate PPE (gloves, lab coat, safety glasses)
- Avoid generating aerosols during handling
- Work in biosafety cabinet when handling concentrated solutions
- Dispose of according to institutional biosafety guidelines
- No special biohazard classification for purified peptide
Frequently Asked Questions
CAMP promoter. Binding of 1,25-dihydroxyvitamin D₃ to the vitamin D receptor induces LL-37 transcription, linking nutritional status to antimicrobial readiness[4].
Clinical Trials & Human Evidence Landscape
Recent LL-37 studies range from interventional oncology and wound-healing trials to observational biomarker investigations in oral and periodontal disease. Registered trials with publicly available protocols are summarised below.
Interventional Studies
Intratumoral LL-37 for Melanoma (NCT02225366)
- Sponsor / Site: MD Anderson Cancer Center, USA[13]
- Design: Phase I dose-escalation with weekly intratumoral LL-37 for eight weeks; 2 participants per cohort, up to four dose levels (planned n=36).
- Population: Adults with injectable melanoma lesions refractory to standard therapy.
- Primary Endpoint: Optimal Biological Dose based on CTCAE dose-limiting toxicities.
- Secondary Endpoints: Immune-related response (irCR/irPR), T-cell phenotyping, radiologic response every 8 weeks.
- Status: Completed; results pending peer-reviewed publication.
Topical LL-37 Cream for Diabetic Foot Ulcers (NCT04098562)
- Sponsor: Universitas Indonesia[14]
- Design: Randomised, placebo-controlled pilot (n=40) delivering 0.5 mg/mL LL-37 cream plus standard wound care twice weekly for four weeks.
- Primary Outcomes: Granulation index (ImageJ) and qualitative aerobic bacterial culture at weeks 1–4.
- Secondary Outcomes: Wound area reduction, cytokine profiling (IL‑1α, TNF‑α).
- Status: Not yet recruiting (last verified September 2019).
Periodontal & Oral Health Studies
Vitamin D & LL-37 Around Implants (NCT06867250)
- Sponsor: Altınbaş University, Türkiye[15]
- Design: Cross-sectional analysis of 33 patients (105 implants) spanning peri-implant health, mucositis, and peri-implantitis.
- Assessments: Clinical indices (mPI, mSBI, probing depth, keratinised mucosa width) and peri-implant sulcus fluid LL-37/vitamin D via ELISA.
- Status: Completed December 2021; statistical analysis underway.
Passive Smoking & Salivary LL-37 in Children (NCT03639376)
- Sponsor: Kırıkkale University, Türkiye[16]
- Design: Observational cohort of 180 children (90 exposed vs. 90 unexposed household smoke).
- Primary Measure: Salivary LL-37 concentration across an 18-month sampling window.
- Secondary Measure: Salivary cotinine to validate passive smoke exposure.
- Status: Completed (2018); awaiting disseminated results.
Smoking, Vitamin D₃ & Periodontal LL-37 (NCT03923218)
- Sponsor: Gazi University, Türkiye[17]
- Design: Case-control (n=60) comparing smokers with chronic periodontitis, non-smokers with periodontitis, and healthy controls.
- Endpoints: Gingival crevicular fluid LL-37 (ELISA), serum vitamin D₃ (HPLC), periodontal parameters (PD, CAL, GI, PI).
- Status: Completed; manuscript in preparation.
LL-37 in Oral Potentially Malignant Lesions (NCT06219330)
- Sponsor: Fayoum University, Egypt[18]
- Design: Case-control (n=45) including healthy subjects, oral lichen planus, and leukoplakia.
- Primary Outcomes: Salivary LL-37 levels, ROC characteristics for lesion discrimination.
- Status: Completed November 2023; results posted January 2024.
Additional Registered Studies
- Skin Barrier & Surfactants (NCT01951352): Interventional crossover (n=10) tracking LL-37 expression after washing with different soaps using tape-stripping timepoints at 5 min, 4 h, and 24 h[19].
- Periodontal Therapy Biomarkers (NCT04404335): Prospective study relating LL-37, IL-10, and TGF-β changes to periodontal treatment response[20].
- Passive Smoking, Oxidative Stress & LL-37 (NCT04292548): Observational study connecting salivary LL-37 and oxidative indices with pediatric passive smoking exposure[21].
References & Scientific Citations
The information provided on this page is supported by peer-reviewed scientific research. Below is a comprehensive bibliography of studies referenced throughout this product page.
Research Integrity:
All claims made on this page are backed by published scientific literature. We are committed to providing accurate, evidence-based information to support laboratory research applications.
- Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 2004;75(1):39-48. PMID: 12960280
- Dürr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758(9):1408-1425. PMID: 16716248
- Agerberth B, et al. The human antimicrobial and chemotactic peptides LL-37 and α-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96(9):3086-3093. PMID: 11049988
- Wang TT, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173(5):2909-2912. PMID: 15322146
- Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol. 2008;122(2):261-266. PMID: 18439663
- Yamasaki K, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13(8):975-980. PMID: 17676051
- Mookherjee N, Hancock REW. Cationic host defence peptides: innate immune regulatory peptides as a novel approach for treating infections. Cell Mol Life Sci. 2007;64(7-8):922-933. PMID: 17310278
- Turner J, et al. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother. 1998;42(9):2206-2214. PMID: 9736536
- Overhage J, et al. Human host defence peptide LL-37 prevents bacterial biofilm formation. Infect Immun. 2008;76(9):4176-4182. PMID: 18591225
- Vandamme D, Landuyt B, Luyten W, Schoofs L. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell Immunol. 2012;280(1):22-35. PMID: 23246832
- Heilborn JD, et al. The cathelicidin antimicrobial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol. 2003;120(3):379-389. PMID: 12603850
- van der Does AM, et al. The human cathelicidin LL-37 is a multifunctional modulator of innate immune responses. J Immunol. 2010;185(10):7084-7093. PMID: 20952669
- ClinicalTrials.gov. Intratumoral Injections of LL37 for Melanoma. Identifier: NCT02225366. Updated December 9, 2021. https://clinicaltrials.gov/study/NCT02225366
- ClinicalTrials.gov. The Efficacy of LL-37 Cream on Aerobic Bacteria Colonization Pattern, Inflammation Response, and Healing Rate of Diabetic Foot Ulcers. Identifier: NCT04098562. Verified September 2019. https://clinicaltrials.gov/study/NCT04098562
- ClinicalTrials.gov. Peri-implant Vitamin D and Cathelicidin (LL-37) Levels. Identifier: NCT06867250. Updated March 10, 2025. https://clinicaltrials.gov/study/NCT06867250
- ClinicalTrials.gov. Passive Smoking and LL-37 in Children. Identifier: NCT03639376. Updated August 21, 2018. https://clinicaltrials.gov/study/NCT03639376
- ClinicalTrials.gov. Effects of Smoking and Vitamin D3 on the Levels of Human Cathelicidin Peptide LL-37. Identifier: NCT03923218. Updated April 22, 2019. https://clinicaltrials.gov/study/NCT03923218
- ClinicalTrials.gov. Cathelicidin LL-37 Relation to Potentially Malignant Lesions. Identifier: NCT06219330. Updated January 23, 2024. https://clinicaltrials.gov/study/NCT06219330
- ClinicalTrials.gov. Effects of Surfactants on the Innate Immune System. Identifier: NCT01951352. Updated October 2, 2019. https://clinicaltrials.gov/study/NCT01951352
- ClinicalTrials.gov. The Role of Anti-inflammatory Cytokines and Antimicrobial Peptide LL-37 Biomarkers in the Treatment of Periodontal Disease. Identifier: NCT04404335. Updated September 30, 2022. https://clinicaltrials.gov/study/NCT04404335
- ClinicalTrials.gov. Salivary TAS, TOS, LL-37 and Dental Status in Passive Smoking Children. Identifier: NCT04292548. Updated March 6, 2020. https://clinicaltrials.gov/study/NCT04292548
Third-Party Testing Results
All products undergo rigorous third-party HPLC (High-Performance Liquid Chromatography) testing to verify purity and quality.
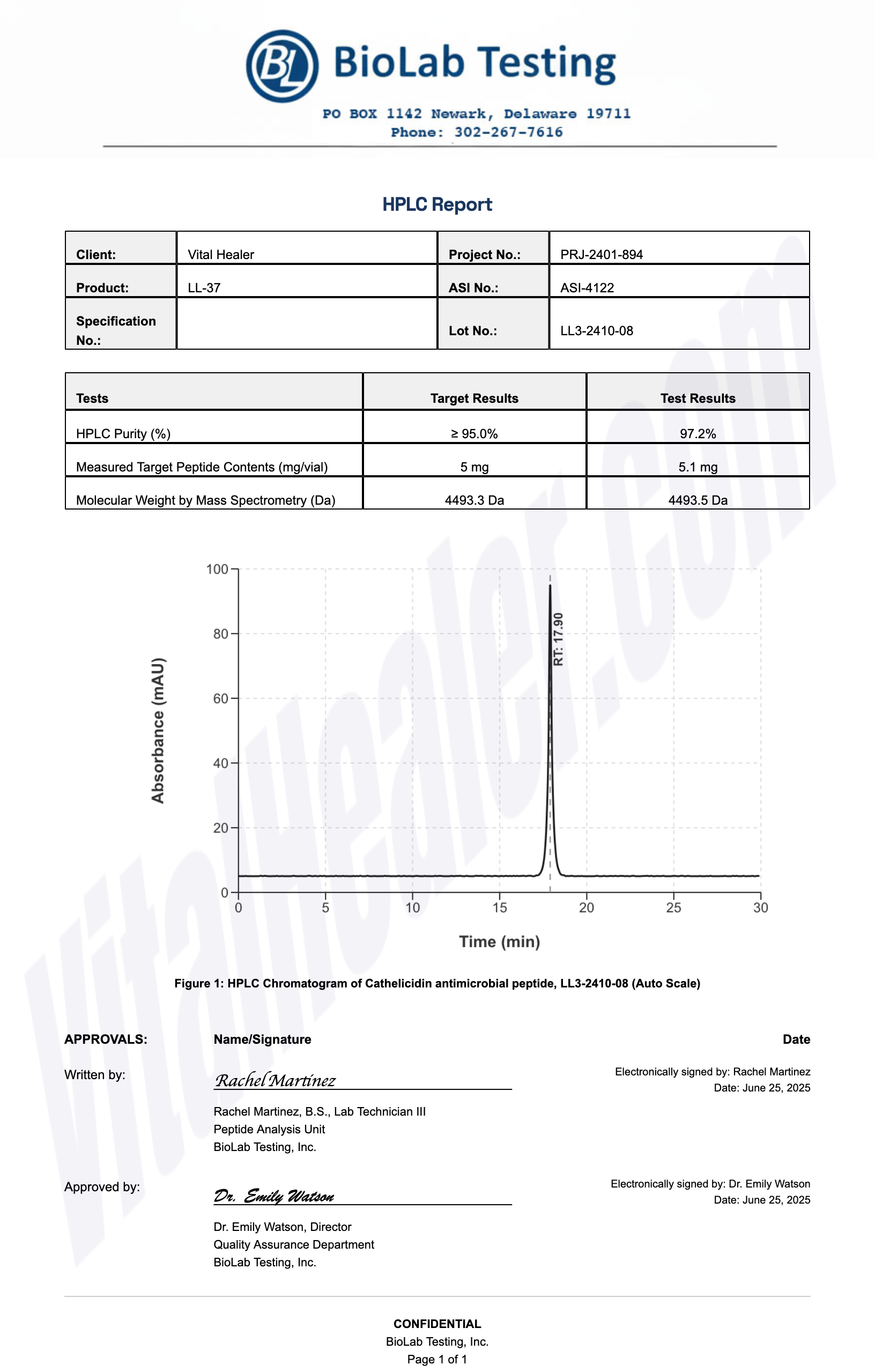
About HPLC Testing:
High-Performance Liquid Chromatography (HPLC) is a standard analytical technique used to verify peptide purity. Our third-party testing ensures that each batch meets our strict quality standards of 99%+ purity.
Related Research Peptides