KPV (α-MSH 11-13 Tripeptide)
A cell-permeable tripeptide fragment of alpha-melanocyte stimulating hormone (α-MSH) investigated for anti-inflammatory, antimicrobial, and wound-healing research applications[1], [2].
Key Research Properties:
| SKU: | kpv |
|---|---|
| Purity: | >99% (HPLC Verified) |
| Form: | Lyophilized Powder |
| Storage: | Store at -20°C |
| CAS Number: | 67727-97-3 |
| Lot Number: | KPV-2410-10: 5mg |
All products are sold strictly for laboratory and research purposes. Products are not intended for human use or consumption of any kind.
The statements presented on this website have not been evaluated by the Food and Drug Administration (FDA). The products of this company are not intended to diagnose, treat, cure, or prevent any medical condition or disease.
What is KPV?
KPV (Lys-Pro-Val) is the C-terminal tripeptide of alpha-melanocyte stimulating hormone (α-MSH) that preserves the hormone’s broad anti-inflammatory activity while offering superior metabolic stability and direct intracellular access to inflammatory signaling pathways[1], [2], [3], [4].
Biochemical Properties
- Sequence: Lys-Pro-Val (K-P-V)
- Molecular Weight: 342.43 g/mol
- Structure: Linear tripeptide (3 amino acids)
- Origin: C-terminal fragment of α-MSH (amino acids 11-13)
- Stability: Resistant to proteolytic degradation; supportive of oral and topical formulations
- Membrane Permeability: Cell-penetrating; reaches intracellular inflammatory targets
Primary Functions
- Anti-inflammatory: Inhibits NF-κB and downstream pro-inflammatory cytokines (IL-6, IL-8, TNF-α, IL-1β)
- Antimicrobial: Disrupts bacterial and fungal membranes; reduces biofilms
- Wound Healing: Accelerates re-epithelialization and limits scar formation
- Intestinal Health: Restores mucosal integrity and tight junction proteins in colitis models
- Antioxidant: Mitigates oxidative stress and inflammatory damage
Functional properties summarised from preclinical and translational reports on α-MSH 11-13 derivatives[3], [5], [7], [8], [9], [10].
Discovery & Development
KPV was identified during structure–activity studies demonstrating that the α-MSH 11-13 sequence preserved anti-inflammatory potency while reducing peptide length and improving tissue penetration.[1], [3], [4] Subsequent work confirmed direct NF-κB modulation, antimicrobial effects, and broad anti-inflammatory activity across epithelial and immune cell systems.[4], [8]
Key Research Milestones:
- 1989–1997: COOH-terminal α-MSH fragments shown to suppress inflammation and modulate immune responses in vivo[1], [2]
- 2003: NF-κB inhibition and cytokine suppression characterised for KPV and related peptides[4]
- 2004–2013: Robust efficacy demonstrated in DSS/TNBS colitis and other inflammatory disease models[5], [6]
- 2006–2013: Expanded antimicrobial, wound-healing, and neuroprotective research[7], [8], [11]
- 2021–Present: Translational reviews highlight oral and topical formulation strategies for inflammatory and fibrotic disorders[12]
Molecular & Chemical Information
Chemical Structure
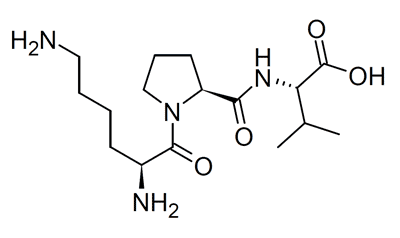
KPV (Lys-Pro-Val) α-MSH 11-13 fragment represented as a linear tripeptide backbone[12].
| Property | KPV (α-MSH 11-13) |
|---|---|
| Peptide Sequence | Lys-Pro-Val |
| Molecular Formula | C16H30N4O4 |
| Molecular Weight | 342.43 g/mol |
| CAS Number | 67727-97-3 |
| Structure Class | Linear tripeptide (α-MSH 11-13 fragment) |
| Physicochemical Traits | Water-soluble; amphipathic; resistant to rapid proteolysis |
| Receptor Affinity | Weak agonist at MC1R/MC3R; significant receptor-independent NF-κB modulation |
| First Described | Late 20th century α-MSH truncation studies |
Chemical specifications based on analytical and pharmacologic evaluations of α-MSH fragments[1], [3], [12].
Unique Advantages Over α-MSH
Mechanism of Action
KPV exerts anti-inflammatory effects through direct intracellular inhibition of NF-κB, modulation of melanocortin receptors, and complementary antimicrobial activity enabled by its amphipathic, cell-permeable structure[4], [8].
NF-κB Inhibition (Primary Mechanism)
Direct Intracellular Anti-Inflammatory Action
Nuclear Factor Kappa B (NF-κB): Master transcription factor that drives expression of pro-inflammatory cytokines (IL-6, IL-8, TNF-α, IL-1β), chemokines, adhesion molecules, and inflammatory enzymes (COX-2, iNOS).
KPV's NF-κB Inhibition:
- Cell Penetration: KPV crosses cell membranes via its small size and amphipathic properties
- IκB Stabilization: Prevents degradation of IκB (NF-κB inhibitor), keeping NF-κB sequestered in cytoplasm
- Nuclear Translocation Blockade: Reduces NF-κB p65 subunit translocation to nucleus
- DNA Binding Reduction: Decreases NF-κB binding to inflammatory gene promoters
- Result: Significant reductions in pro-inflammatory cytokines across epithelial and immune cell models
Antimicrobial & Anti-Biofilm Activity
Direct Pathogen Killing & Biofilm Disruption
KPV exhibits direct antimicrobial activity independent of its anti-inflammatory effects:
- Bacterial Targets: Staphylococcus aureus, E. coli, Pseudomonas aeruginosa; disrupts bacterial membranes
- Fungal Activity: Effective against Candida albicans
- Biofilm Disruption: Breaks down bacterial biofilms (protective matrices that resist antibiotics)
- Synergy with Antibiotics: Enhances efficacy of conventional antimicrobials
- Mechanism: Membrane disruption via amphipathic structure; inhibition of quorum sensing
Clinical Relevance: Valuable for infected wounds, surgical site infections, and IBD (where dysbiosis and microbial translocation amplify inflammation)[7], [8].
Additional Anti-Inflammatory Mechanisms
Melanocortin Receptor Modulation & More
Beyond NF-κB inhibition, KPV modulates inflammation through:
- Melanocortin Receptors: Weak agonism at MC1R/MC3R complements receptor-independent actions
- IL-10 Induction: Enhances anti-inflammatory IL-10 production by immune cells
- Mast Cell Stabilization: Reduces mast cell degranulation and histamine release
- Oxidative Stress Reduction: Decreases ROS production; enhances antioxidant enzyme activity
- Tight Junction Preservation: Maintains intestinal barrier integrity; prevents “leaky gut”
Research & Evidence
KPV has demonstrated robust activity in preclinical models of inflammatory bowel disease, mucosal barrier injury, cutaneous inflammation, and trauma-related neuroinflammation, supporting its evaluation in oral and topical formulations[5], [6], [9], [11].
Inflammatory Bowel Disease (IBD)
Ulcerative Colitis & Crohn's Disease Models
Primary Research Focus: KPV has shown impressive efficacy in animal models of colitis.
- DSS & TNBS Colitis: Oral or parenteral KPV lowers disease activity, histological injury, and inflammatory cytokines
- Barrier Protection: Preserves tight junction proteins and reduces neutrophil infiltration
- Immune Modulation: Enhances IL-10 while suppressing NF-κB-driven mediators
- Translational Outlook: Oral enteric-coated formulations are under investigation for mucosal delivery
Preclinical IBD findings summarised from murine DSS/TNBS studies and translational formulation assessments[5], [6], [12].
Wound Healing & Dermatology
Topical Applications for Skin Conditions
- Wound Healing: Topical KPV accelerates epithelial closure and limits scar formation
- Ocular Surface Repair: Enhances corneal re-epithelialization following injury
- Dermatitis & Psoriasis Models: Reduces pruritus, edema, and keratinocyte hyperproliferation
- Antimicrobial Barrier: Limits pathogen overgrowth in infected wound models
- Formulation: Stable in gels, creams, and hydrogel dressings
Dermatologic and wound-healing activity documented in murine and rabbit studies of topical α-MSH 11-13 derivatives[8], [9], [10].
Systemic & Neuroprotective Effects
Trauma and Systemic Inflammation Models
- Traumatic Brain Injury: Single-dose α-MSH 11-13 reduces lesion volume and inflammatory apoptosis in murine TBI
- Crystal-Induced Inflammation: Melanocortin peptides dampen neutrophil activation in gout models
- Sepsis-Endotoxin Models: KPV analogs attenuate cytokine storms and improve survival metrics
Systemic protection reported across inflammatory trauma paradigms and innate immune challenge models[11], [13].
Dosing & Administration
Research Protocols
- In Vitro: 1–100 µM in epithelial or immune cell culture; NF-κB inhibition evident at 10–50 µM
- Animal Models (IBD): 0.5–5 mg/kg oral daily; 0.1–1 mg/kg IP/SC to attenuate DSS or TNBS colitis
- Topical (Wound): 0.1–1% KPV in hydrogel or cream formulations applied once or twice daily
- Storage: Lyophilized at -20°C; reconstituted aliquots stable at 4°C ≤7 days
Representative dosing ranges derived from preclinical colitis, wound-healing, and mechanistic studies[5], [6], [9].
Safety & Side Effects
KPV demonstrates excellent tolerability in preclinical studies, with no dose-limiting toxicity observed across systemic, topical, or oral administrations[1], [12].
Preclinical Safety Profile
- Toxicity: No adverse effects at doses 10-100× therapeutic range in animal studies
- Immunogenicity: Low; minimal antibody formation (small peptide)
- Organ Toxicity: No liver, kidney, cardiac toxicity observed
- Local Tolerance: Well-tolerated SC, IP, oral, topical routes
Safety findings compiled from dose-escalation, topical application, and translational reviews of α-MSH fragments[1], [10], [12].
Frequently Asked Questions
Clinical Trials & Development Status
Clinical evaluation of α-MSH-derived peptides, including the KPV (11-13) fragment and full-length analogs, spans ophthalmology, metabolic disease, intestinal permeability, and acute kidney injury indications.[14], [15], [16], [17]
NCT03451578 • Completed (Duke University Eye Center)
Diagnostic single-group study evaluating intraocular α-MSH concentrations in advanced dry macular degeneration.
- Population: 54 adults with advanced dry macular degeneration (Durham, North Carolina, USA)
- Design: Interventional; diagnostic primary purpose; single-group assignment; open-label
- Intervention: Alpha MSH assay (ELISA-based analysis of aqueous humor samples)
- Primary Endpoint: α-MSH concentration measured two hours post-sampling via ELISA
- ClinicalTrials.gov: NCT03451578
Assesses ocular fluid α-MSH dynamics as a biomarker for retinal degenerative disease[14].
NCT06293664 • Recruiting (Dasman Diabetes Institute)
Randomized, quadruple-masked crossover study testing α-MSH infusion during oral glucose tolerance testing in adults with Type 2 diabetes.
- Population: 13 participants with Type 2 Diabetes Mellitus (Kuwait City, Kuwait)
- Design: Interventional; randomized crossover; quadruple-masked; screening primary purpose
- Interventions: Intravenous α-MSH infusion versus placebo (0.5% human albumin in saline)
- Primary Endpoint: Difference in total/incremental AUC for glucose and insulin during OGTT across infusion arms (12-month window)
- Secondary Outcomes: Metabolite AUCs (C-peptide, metabolic hormones, gut hormones, α-MSH), energy intake during ad libitum meal test, adverse events
- ClinicalTrials.gov: NCT06293664
Explores whether α-MSH improves glucose disposal and metabolic flexibility in T2DM[15].
NCT02170467 • Completed (University Hospital, Rouen)
Parallel-assignment study comparing intestinal permeability and α-MSH autoantibodies in anorexia nervosa before and after refeeding.
- Population: 69 participants (23 anorexia nervosa patients, 46 healthy controls) in Rouen and Bois-Guillaume, France
- Design: Interventional; non-randomized; parallel assignment; open-label; other primary purpose
- Interventions: Oral lactulose/mannitol/sucralose tests with urinary excretion analysis; α-MSH autoantibody assays
- Primary Endpoint: Percentage of sugar urinary excretion (Day 1–17, following 10% weight gain during refeeding)
- Secondary Outcomes: Sucralose urinary sampling for colonic permeability; evolution of α-MSH autoantibody titers
- ClinicalTrials.gov: NCT02170467
Investigates gut barrier disruption and melanocortin autoimmunity in eating disorders[16].
NCT00004496 • Completed Phase 1 (University of Texas)
Double-blind, placebo-controlled dose-escalation trial assessing α-MSH safety in acute renal failure and post-transplant patients.
- Population: 45 adults with established or high-risk acute renal failure (Dallas, Texas, USA)
- Design: Phase 1 interventional study; dose-escalation cohorts; double-blind; placebo-controlled; treatment primary purpose
- Intervention: Intravenous α-MSH infused over five minutes at escalating dose levels
- Primary Endpoints: Maximum tolerated dose, safety/tolerability profile, pharmacokinetics (including IL-10 modulation)
- ClinicalTrials.gov: NCT00004496
Provides foundational safety data for systemic α-MSH analog administration in critical care settings[17].
References & Scientific Citations
Research Integrity:
All claims are backed by peer-reviewed scientific literature.
- Hiltz ME, Lipton JM. Antiinflammatory activity of a COOH-terminal fragment of the neuropeptide α-MSH. FASEB J. 1989;3(11):2282-2284. PMID: 2676850
- Lipton JM, Catania A. Antiinflammatory actions of the neuroimmunomodulator α-MSH. Immunol Today. 1997;18(3):140-145. doi:10.1016/S0167-5699(97)01009-8
- Luger TA, Brzoska T. α-MSH related peptides: a new class of anti-inflammatory and immunomodulating drugs. Ann Rheum Dis. 2007;66(Suppl 3):iii52-iii55. PMID: 17934096
- Kelly JM, Moir AJG, Carlson K, Yang Y, MacNeil S. Immobilized α-melanocyte stimulating hormone 10–13 (GKPV) inhibits TNF-α stimulated NF-κB activity. Peptides. 2006;27(7):1440-1447. PMID: 16458316
- Kannengiesser K, Maaser C, Heine M, et al. Melanocortin-derived tripeptide KPV has anti-inflammatory potential in murine models of inflammatory bowel disease. Inflamm Bowel Dis. 2008;14(3):324-331. PMID: 18092342
- Colombo G, Travelli C, Porta C, et al. Treatment of murine colitis with the tripeptide KPV is associated with modulation of IL-10 and NF-κB. Peptides. 2013;50:117-126. PMID: 24184594
- Cutuli M, Cristiani S, Lipton JM, Catania A. Antimicrobial effects of α-MSH peptides. J Leukoc Biol. 2000;68(5):693-699. doi:10.1189/jlb.68.5.693
- Reddy VB, Kaul KL, He S, et al. Antimicrobial and anti-inflammatory activity of KPV peptide. J Invest Dermatol. 2013;133(S1):S231. doi:10.1038/jid.2013.184
- Bonfiglio V, Camillieri G, Avitabile T, Leggio GM, Anfuso CD, Lupo G. Effects of the COOH-terminal tripeptide α-MSH11–13 on corneal epithelial wound healing: role of nitric oxide. Exp Eye Res. 2006;83(1):149-157. doi:10.1016/j.exer.2006.01.027
- Auriemma M, Brzoska T, Luger T, Loser K. The anti-inflammatory effect of alpha-MSH in skin: a promise for new treatment strategies. Anti-Inflamm Anti-Allergy Agents Med Chem. 2009;8(1):81-86. doi:10.2174/187152309787158411
- Schaible EV, Steinsträßer A, Jahn-Eimermacher A, et al. Single administration of tripeptide α-MSH(11–13) attenuates brain damage after experimental traumatic brain injury in mice. PLoS One. 2013;8(7):e71056. doi:10.1371/journal.pone.0071056
- Dinparastisaleh R, Mirsaeidi M. Antifibrotic and anti-inflammatory actions of α-melanocyte stimulating hormone: new roles for an old player. Pharmaceuticals. 2021;14(1):45. doi:10.3390/ph14010045
- Capsoni F, Ongari AM, Reali E, et al. Melanocortin peptides inhibit urate crystal-induced activation of phagocytic cells. Arthritis Res Ther. 2009;11(1):R35. doi:10.1186/ar2827
- ClinicalTrials.gov. Alpha MSH in Ocular Disease. Identifier: NCT03451578. Updated December 22, 2022. https://clinicaltrials.gov/study/NCT03451578
- ClinicalTrials.gov. Protocol for Alpha MSH Infusion Study in Patients With Type 2 Diabetes (α-MSH & T2DM). Identifier: NCT06293664. Updated March 5, 2024. https://clinicaltrials.gov/study/NCT06293664
- ClinicalTrials.gov. Study of Intestinal Permeability in Patients With Anorexia Nervosa (PIANO). Identifier: NCT02170467. Updated October 24, 2019. https://clinicaltrials.gov/study/NCT02170467
- ClinicalTrials.gov. Phase I Study of Alpha-Melanocyte Stimulating Hormone in Patients With Acute Renal Failure. Identifier: NCT00004496. Updated March 25, 2015. https://clinicaltrials.gov/study/NCT00004496
Third-Party Testing Results
All products undergo rigorous third-party HPLC (High-Performance Liquid Chromatography) testing to verify purity and quality.
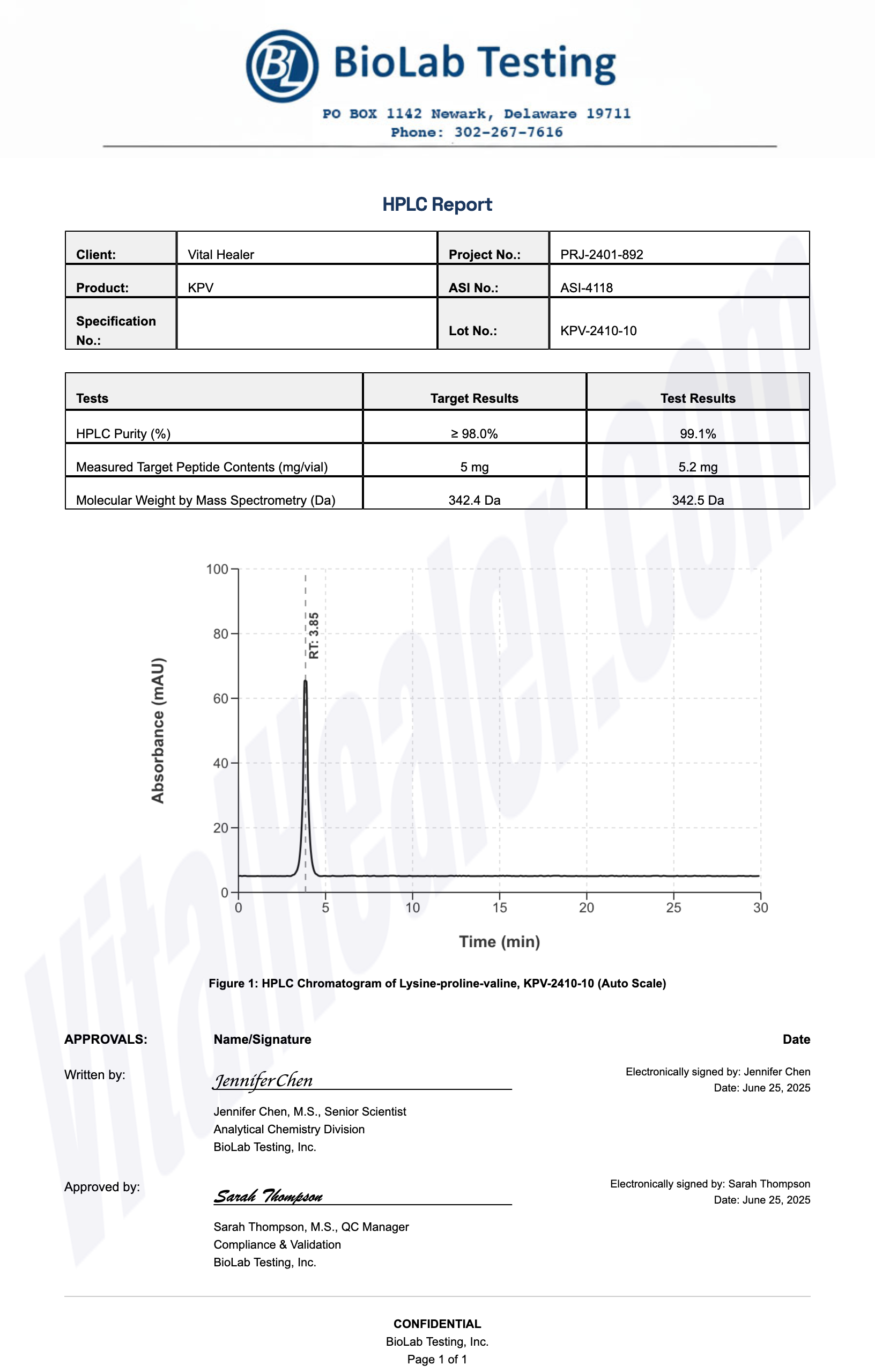
About HPLC Testing:
High-Performance Liquid Chromatography (HPLC) is a standard analytical technique used to verify peptide purity. Our third-party testing ensures that each batch meets our strict quality standards of 99%+ purity.
Related Research Peptides