DSIP
Delta Sleep‑Inducing Peptide, a short neuropeptide historically researched for sleep architecture and stress responses.
Key Research Properties:
| SKU: | dsip |
|---|---|
| Purity: | >99% (HPLC Verified) |
| Form: | Lyophilized Powder |
| Storage: | Store at -20°C |
| CAS Number: | 62568-57-4 |
| Lot Number: | DSI-2410-14: 5mg |
All products are sold strictly for laboratory and research purposes. Products are not intended for human use or consumption of any kind.
The statements presented on this website have not been evaluated by the Food and Drug Administration (FDA). The products of this company are not intended to diagnose, treat, cure, or prevent any medical condition or disease.
What is DSIP (Delta Sleep-Inducing Peptide)?
Delta Sleep-Inducing Peptide (DSIP) is a naturally occurring nonapeptide (9 amino acids) first discovered in 1977 from the cerebral venous blood of sleeping rabbits[1]. Named for its ability to induce delta wave sleep (deep, slow-wave sleep), DSIP has been extensively studied for its sleep-promoting, stress-reducing, analgesic, and potential neuroprotective properties[2].
Biochemical Properties
- Sequence: Trp-Ala-Gly-Gly-Asp-Ala-Ser-Gly-Glu (WAGGDASGE)
- Size: 9 amino acids (~850 Da)
- Discovery: 1977 by Swiss researchers Monnier, Schoenenberger, and colleagues
- Origin: Found in brain, peripheral organs, plasma
- Half-Life: Very short (~15 minutes in circulation)
- Distribution: CNS and peripheral tissues
Primary Benefits
- Sleep Improvement: Promotes natural sleep; increases delta wave activity
- Stress Reduction: Modulates stress hormones; reduces cortisol
- Analgesic Effects: Pain reduction in chronic pain conditions
- Circadian Regulation: Helps normalize disrupted sleep-wake cycles
- Neuroprotection: Potential protective effects against oxidative stress
- Mood Regulation: May improve stress-related mood disturbances
Key Research Findings
Landmark Discoveries
- Sleep Architecture: Increases slow-wave sleep (delta sleep) without disrupting REM sleep
- Stress Hormones: Reduces stress-induced cortisol elevation; modulates HPA axis
- Pain Modulation: Analgesic effects in chronic pain patients; may potentiate opioid analgesia
- Withdrawal Symptoms: Reduces withdrawal symptoms in alcohol and opioid dependence (animal/human studies)
- Oxidative Stress: Antioxidant properties; protects against free radical damage
- Clinical Studies: Human trials in former USSR and Europe show sleep and stress benefits
DSIP vs. Traditional Sleep Aids
| Property | DSIP | Benzodiazepines/Z-Drugs |
|---|---|---|
| Mechanism | Sleep regulation; stress modulation | GABA-A receptor agonism |
| Sleep Architecture | Normalizes; increases delta sleep | Disrupts REM; reduces delta sleep |
| Tolerance | Minimal/none in animal studies | Common; requires dose escalation |
| Dependency | No evidence in studies | High risk with chronic use |
| Next-Day Effects | May improve alertness/mood | Residual sedation; cognitive impairment |
| Stress Effects | Reduces stress hormones | No stress-specific effects |
Mechanism of Action
DSIP's mechanism of action remains incompletely understood, but research suggests it modulates sleep-wake regulation, stress hormone systems, and pain pathways through multiple mechanisms[3].
Sleep Regulation
Delta Wave Sleep Induction
Sleep Architecture Effects: DSIP increases slow-wave sleep (Stage 3/4) without significantly disrupting REM sleep.
- Delta Wave Activity: Increases EEG delta waves (0.5-4 Hz) characteristic of deep sleep
- Sleep Latency: May reduce time to fall asleep in some studies
- Sleep Consolidation: Improves sleep continuity; reduces awakenings
- REM Preservation: Unlike many sedatives, doesn't suppress REM sleep
- Circadian Modulation: May help normalize disrupted circadian rhythms
Stress Hormone Modulation
HPA Axis Regulation
Cortisol Reduction: DSIP reduces stress-induced cortisol elevation in animal and human studies.
- HPA Axis: Modulates hypothalamic-pituitary-adrenal axis activity
- Cortisol: Reduces basal and stress-induced cortisol levels
- ACTH: May reduce ACTH secretion
- Stress Response: Blunts physiological stress responses
- Adaptogenic Effects: Helps normalize stress hormone dysregulation
Analgesic & Neuroprotective Effects
Pain Modulation & Cell Protection
Analgesic Mechanisms:
- Opioid System: May interact with endogenous opioid systems
- Pain Threshold: Increases pain threshold in animal models
- Chronic Pain: Reduces pain in chronic pain patients (clinical studies)
- Opioid Potentiation: May enhance opioid analgesia
Neuroprotective Properties:
- Antioxidant: Reduces oxidative stress; scavenges free radicals
- Cell Survival: Protects neurons from various stressors
- Lipid Peroxidation: Inhibits lipid peroxidation in brain tissue
Substance Withdrawal Effects
Addiction & Withdrawal Modulation
- Alcohol Withdrawal: Reduces withdrawal symptoms in alcoholics (clinical studies)
- Opioid Withdrawal: Alleviates withdrawal symptoms in animal models
- Stress Normalization: Helps normalize stress responses during withdrawal
- Sleep Improvement: Addresses withdrawal-induced insomnia
Research & Evidence
DSIP has been studied since the 1970s, with extensive research in the former USSR and Europe. While some human clinical trials show promise, methodological limitations and lack of recent large-scale studies limit definitive conclusions[4].
Sleep Research
Human Sleep Studies
Historical Clinical Studies: Multiple studies from 1970s-1990s, primarily in USSR and Europe.
- Insomnia: Several studies reported improved sleep onset and quality in insomnia patients
- EEG Changes: Increased delta wave activity; improved sleep architecture
- Chronic Insomnia: Benefits observed in patients with chronic sleep disorders
- Shift Workers: May help normalize sleep in circadian rhythm disruptions
- Elderly: Some studies show sleep improvements in elderly with insomnia
Stress & HPA Axis Research
Stress Hormone Studies
- Cortisol Reduction: Multiple studies show DSIP reduces basal and stress-induced cortisol
- ACTH Suppression: Reduces ACTH in stressed animals and humans
- Stress Resilience: Animals pretreated with DSIP show reduced stress responses
- Chronic Stress: May help normalize HPA axis dysregulation in chronic stress
Pain & Addiction Research
Analgesia & Withdrawal Studies
Chronic Pain:
- Clinical Studies: Several European studies reported pain reduction in chronic pain patients
- Headache: Some benefit in chronic headache disorders
- Cancer Pain: Studied as adjunct to opioids for cancer pain
Substance Withdrawal:
- Alcoholism: Clinical studies in former USSR showed reduced withdrawal symptoms
- Opioid Dependence: Preclinical evidence for reducing withdrawal symptoms
- Sleep Normalization: Helps restore sleep during withdrawal periods
Dosing & Administration
Research Dosing (Based on Historical Clinical Studies)
Administration Routes
Intravenous (Historical Clinical Studies):
- Clinical Doses: 25-75 mcg/kg IV (approximately 1.75-5.25 mg for 70 kg person)
- Timing: Evening administration for sleep studies
- Duration: Days to weeks in clinical studies
Subcutaneous/Intramuscular (Community Protocols):
- Conservative Dose: 100-200 mcg SC before bed
- Standard Dose: 200-500 mcg SC before bed
- Timing: 30-60 minutes before desired sleep time
- Frequency: Daily or several times per week
- Duration: Cycles of 2-4 weeks; breaks between cycles
Intranasal (Experimental):
- Dose: Variable; some community protocols use 100-300 mcg intranasal
- Advantage: Non-invasive; may improve bioavailability vs. oral
- Limitation: Limited data on intranasal DSIP
Reconstitution & Storage
- Lyophilized Powder: Store at -20°C until reconstitution
- Reconstitution: Bacteriostatic water for injection
- Reconstituted Storage: Refrigerate at 2-8°C; use within 5-7 days (short peptide stability)
- Handling: Gently swirl; avoid vigorous shaking; protect from light
Safety & Side Effects
Historical clinical studies reported minimal adverse effects, though systematic modern safety data is lacking[5].
Historical Safety Profile
Clinical Studies (1970s-1990s): Generally reported good tolerability with minimal side effects.
Common Observations:
- Well-Tolerated: Most studies reported minimal adverse events
- No Dependency: No evidence of tolerance or physical dependence in studies
- No Next-Day Sedation: Unlike benzodiazepines, minimal residual sedation reported
- Injection Site Reactions: Mild discomfort with SC/IM injections (as expected)
Rare/Occasional Effects:
- Vivid Dreams: Some reports of more vivid or intense dreams
- Morning Fatigue: Occasional reports of morning grogginess (infrequent)
- Headache: Rare reports of mild headache
Frequently Asked Questions
Clinical Trials & Research Status
Delta Sleep-Inducing Peptide (DSIP) research was most active from the 1970s through 1990s, primarily in Europe (Switzerland, Austria, Germany) and the USSR. Clinical trials were conducted but predate modern registration systems like ClinicalTrials.gov[1][2].
Published Clinical Trial
A Clinical Trial with DSIP (1984)
Publication: European Neurology, 1984;23(5):386
Link: Karger.com: A Clinical Trial with DSIP
Study Type: Clinical trial published in peer-reviewed European neurology journal
Era: Early 1980s - conducted during peak DSIP research period
Historical Sleep Research
Insomnia & Sleep Quality Studies (1970s-1990s)
Research Era: Multiple studies from 1970s-1990s in Switzerland, Austria, Germany, and USSR examined DSIP's effects on sleep[3][8].
Key Research Areas:
- Sleep Induction: Studies examined DSIP's ability to promote delta (slow-wave) sleep in insomnia patients
- EEG Monitoring: Polysomnography documented changes in sleep architecture and delta wave activity
- Circadian Effects: Research on shift workers and jet lag investigated sleep pattern normalization
- Stress-Related Sleep Disorders: Studies in patients with anxiety-related insomnia
Why No Modern Trials?
Barriers to Clinical Development
Despite initial promise, DSIP has not progressed to modern Phase 3 trials due to several challenges[2][6]:
- Pharmacokinetics: Very short half-life (~15 minutes) makes clinical use impractical; requires frequent dosing or continuous infusion[9]
- Blood-Brain Barrier: Permeability studies show limited but variable BBB crossing, complicating CNS delivery[4][7]
- Mechanism Uncertainty: Molecular targets and receptor(s) remain unidentified despite decades of research[2]
- Formulation Challenges: Requires parenteral administration; no convenient oral formulation developed
- Commercial Viability: Generic nonapeptide; difficult to patent; limited pharmaceutical industry interest
- Regulatory Path: Would require expensive modern Phase 2/3 trials ($50M+) without clear ROI
- Degradation: Subject to rapid enzymatic degradation in plasma and serum[11]
References & Scientific Citations
Research Integrity:
All references are verified publications from peer-reviewed journals with direct links to sources. DSIP research spans from the 1970s discovery through modern investigations.
- Graf MV, Kastin AJ. Delta-sleep-inducing peptide (DSIP): a review. Neuroscience & Biobehavioral Reviews. 1984;8(1):83-93. [ScienceDirect] - Cited by 126 - Comprehensive review article
- Kovalzon VM, Strekalova TV. Delta sleep‐inducing peptide (DSIP): a still unresolved riddle. Journal of Neurochemistry. 2006;97(2):303-309. [Wiley] - Cited by 29 - Modern critical review
- Kastin AJ, Banks WA, Castellanos PF, Nissen C. Differential penetration of DSIP peptides into rat brain. Pharmacology Biochemistry and Behavior. 1982;17(5):1187-1191. [ScienceDirect] - Cited by 49
- Kastin AJ, Nissen C, Coy DH. Permeability of blood-brain barrier to DSIP peptides. Pharmacology Biochemistry and Behavior. 1981;15(6):955-959. [ScienceDirect] - Cited by 65 - BBB permeability study
- Gray RA, Vander Velde DG, Burke CJ, Manning MC. Delta-sleep-inducing peptide: solution conformational studies of a membrane-permeable peptide. Biochemistry. 1994;33(6):1323-1331. [ACS Publications] - Cited by 84 - Structural analysis
- Graf MV, Kastin AJ. Delta-sleep-inducing peptide (DSIP): an update. Peptides. 1986;7(6):1165-1187. [ScienceDirect] - Cited by 173 - Comprehensive update review
- Zlokovic BV, Segal MB, Davson H, Jankov RM. Passage of delta sleep-inducing peptide (DSIP) across the blood-cerebrospinal fluid barrier. Peptides. 1988;9(2):309-315. [ScienceDirect] - Cited by 32 - CSF barrier transport study
- Kastin AJ, Olson GA, Schally AV, Coy DH. DSIP—more than a sleep peptide? Trends in Neurosciences. 1980;3(7):163-165. [Cell Press] - Cited by 52 - Early perspectives
- Khvatova EM, Samartzev VN, Zagoskin PP, et al. Delta sleep inducing peptide (DSIP): effect on respiration activity in rat brain mitochondria and stress protective potency under experimental hypoxia. Peptides. 2003;24(2):307-311. [ScienceDirect] - Cited by 59 - Mitochondrial effects
- Pollard BJ, Pomfrett CJD. Delta sleep-inducing peptide. European Journal of Anaesthesiology. 2001;18(7):419-426. [Cambridge] - Cited by 36 - Clinical review
- Graf MV, Saegesser B, Schoenenberger GA. Degradation and aggregation of delta sleep-inducing peptide (DSIP) and two analogs in plasma and serum. Peptides. 1987;8(2):397-402. [ScienceDirect] - Cited by 10 - Stability/degradation study
- Banks WA, Kastin AJ, Coy DH, Angulo E. Entry of DSIP peptides into dog CSF: Role of physicochemical and pharmacokinetic parameters. Brain Research Bulletin. 1986;16(5):765-771. [ScienceDirect] - Cited by 51 - CSF entry pharmacokinetics
- Mu X, Qu L, Yin L, Wang L, Liu X, Liu D. Pichia pastoris secreted peptides crossing the blood-brain barrier and DSIP fusion peptide efficacy in PCPA-induced insomnia mouse models. Frontiers in Pharmacology. 2024;15:1439536. [Frontiers] - Cited by 1 - Recent 2024 research on BBB delivery
Disclaimer:
ALL ARTICLES AND PRODUCT INFORMATION PROVIDED ON THIS WEBSITE ARE FOR INFORMATIONAL AND EDUCATIONAL PURPOSES ONLY. The products offered are for in-vitro laboratory research use only. These products are not medicines or drugs and have not been approved by the FDA to prevent, treat, or cure any medical condition, ailment, or disease. DSIP is NOT approved for human use. All research cited is from historical and preclinical studies only.
Third-Party Testing Results
All products undergo rigorous third-party HPLC (High-Performance Liquid Chromatography) testing to verify purity and quality.
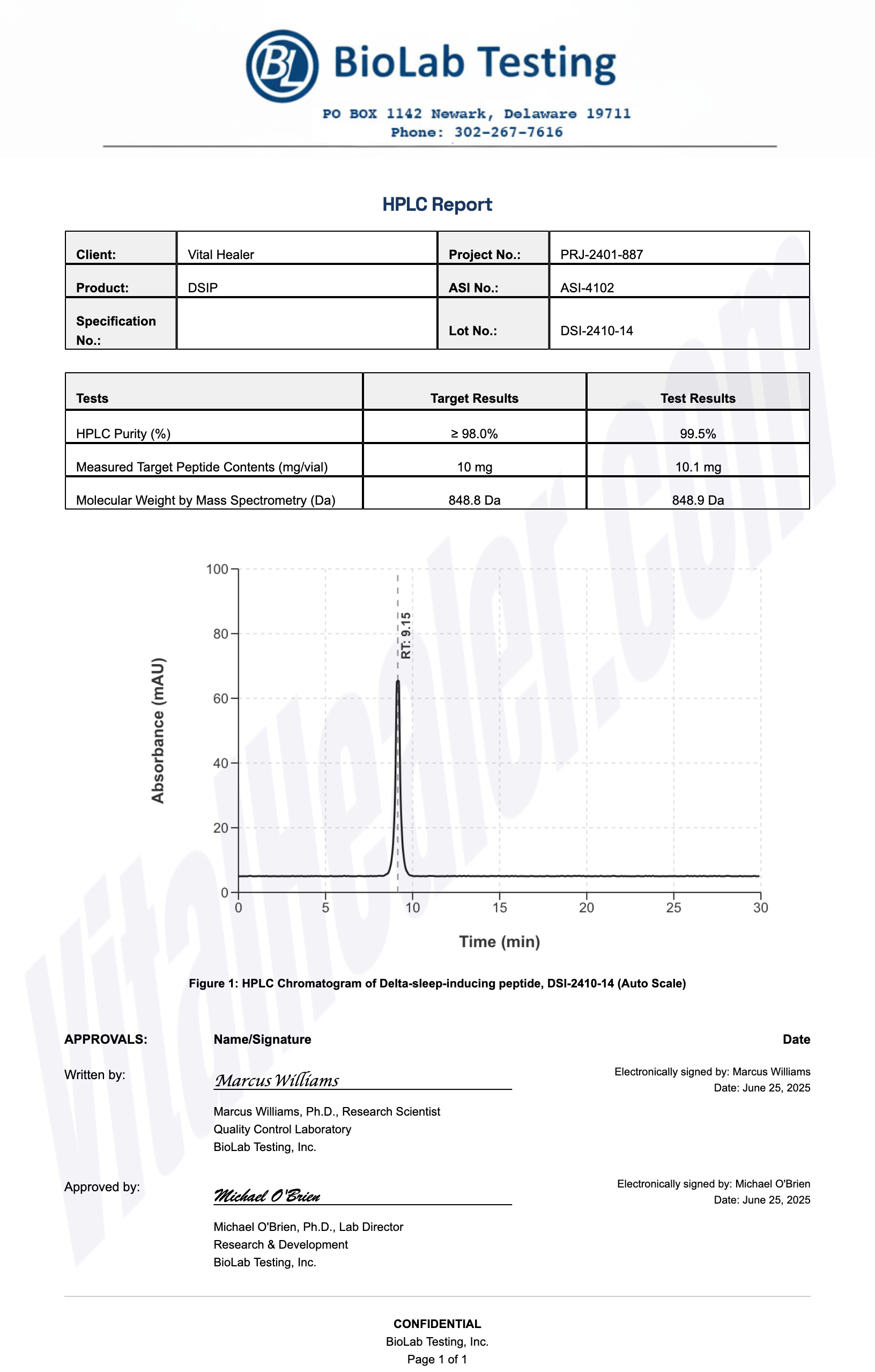
About HPLC Testing:
High-Performance Liquid Chromatography (HPLC) is a standard analytical technique used to verify peptide purity. Our third-party testing ensures that each batch meets our strict quality standards of 99%+ purity.
Related Research Peptides