NAD+
Nicotinamide adenine dinucleotide, a ubiquitous redox cofactor central to cellular metabolism.
Key Research Properties:
| SKU: | nad+ |
|---|---|
| Purity: | >99% (HPLC Verified) |
| Form: | Lyophilized Powder |
| Storage: | Store at -20°C |
| CAS Number: | 53-84-9 |
| Lot Number: | NAD-2410-20: 100mg |
All products are sold strictly for laboratory and research purposes. Products are not intended for human use or consumption of any kind.
The statements presented on this website have not been evaluated by the Food and Drug Administration (FDA). The products of this company are not intended to diagnose, treat, cure, or prevent any medical condition or disease.
Overview
Nicotinamide adenine dinucleotide (NAD⁺) is a fundamental coenzyme present in every living cell, serving as a critical electron carrier in redox reactions and a substrate for numerous enzymes involved in cellular metabolism, DNA repair, gene expression, and stress responses. As organisms age, NAD⁺ levels naturally decline, contributing to mitochondrial dysfunction, metabolic dysregulation, and age-related diseases.[1]
Chemical Structure and Properties
NAD⁺ consists of two nucleotides joined through their phosphate groups, with one nucleotide containing an adenine base and the other containing nicotinamide:
Molecular Specifications
- Molecular Formula: C₂₁H₂₇N₇O₁₄P₂
- Molecular Weight: 663.43 g/mol
- CAS Number: 53-84-9
- Structure: Dinucleotide (adenine + nicotinamide)
- Chemical Name: β-Nicotinamide adenine dinucleotide
Redox Forms
- NAD⁺: Oxidized form (accepts electrons)
- NADH: Reduced form (donates electrons)
- NADP⁺: Phosphorylated oxidized form
- NADPH: Phosphorylated reduced form
- Ratio: NAD⁺/NADH ratio critical for cellular function
NAD⁺ Biosynthesis Pathways
The human body synthesizes NAD⁺ through three distinct pathways, each utilizing different precursor molecules:
Starting Point: L-tryptophan (essential amino acid)
Process: Tryptophan is converted through multiple enzymatic steps to quinolinic acid, which is then converted to nicotinic acid mononucleotide (NAMN), and ultimately to NAD⁺.
Significance: This pathway is energy-intensive and produces only a fraction of cellular NAD⁺ needs. However, it's the only pathway that can create NAD⁺ entirely from scratch.
Limitation: Requires approximately 60 mg of tryptophan to produce 1 mg of NAD⁺, making it inefficient for maintaining cellular NAD⁺ levels.[3]
Starting Point: Nicotinamide (NAM), the recycled product of NAD⁺-consuming reactions
Key Enzyme: Nicotinamide phosphoribosyltransferase (NAMPT) - the rate-limiting enzyme
Process: NAM → Nicotinamide mononucleotide (NMN) → NAD⁺
Significance: This pathway accounts for the majority (>90%) of NAD⁺ synthesis in mammals. NAMPT expression declines with age, contributing to age-related NAD⁺ decline.
Therapeutic Target: Bypassing NAMPT by supplementing with NMN or NR (which enter the salvage pathway downstream of NAMPT) is a key strategy for boosting NAD⁺ levels.[4]
Starting Point: Nicotinic acid (NA, also known as niacin or vitamin B3)
Process: NA → Nicotinic acid mononucleotide (NAMN) → Nicotinic acid adenine dinucleotide (NAAD) → NAD⁺
Significance: This pathway uses dietary niacin (vitamin B3) to produce NAD⁺. It's independent of NAMPT and can bypass age-related salvage pathway decline.
Limitation: High doses of nicotinic acid cause vasodilation and flushing, limiting its use as an NAD⁺ booster.[5]
Age-Related NAD⁺ Decline
One of the most consistent findings in aging biology is the progressive decline in cellular NAD⁺ levels across tissues and organisms. This decline has profound implications for health and longevity:
NAD⁺ Decline: Key Facts
- Magnitude: NAD⁺ levels decline by approximately 50% between ages 20 and 80 in multiple tissues[6]
- Mechanisms: Reduced biosynthesis (decreased NAMPT activity), increased consumption (hyperactivation of PARPs and CD38), and mitochondrial dysfunction
- Consequences: Impaired mitochondrial function, reduced SIRT1 activity, decreased DNA repair, metabolic dysfunction, and increased inflammation
- Reversibility: Animal studies demonstrate that restoring NAD⁺ levels can reverse multiple age-related phenotypes, including metabolic dysfunction, cognitive decline, and muscle atrophy[7]
- Therapeutic Window: Interventions that boost NAD⁺ appear most effective when initiated before severe age-related pathology develops
NAD⁺ Precursors: Comparison
Since direct NAD⁺ supplementation has limited bioavailability, research has focused on NAD⁺ precursors that can be efficiently converted to NAD⁺ in cells:
| Precursor | Pathway | Advantages | Disadvantages | Research Status |
|---|---|---|---|---|
| NR (Nicotinamide Riboside) | Salvage (bypasses NAMPT) | Well-tolerated, oral bioavailable, extensive human safety data | Relatively expensive, degradation in gut | Phase II/III trials |
| NMN (Nicotinamide Mononucleotide) | Salvage (bypasses NAMPT) | Potent NAD⁺ booster, rapidly absorbed | May require dephosphorylation to NR for absorption, fewer human trials | Phase I/II trials |
| Nicotinamide (NAM) | Salvage (NAMPT-dependent) | Inexpensive, widely available, well-studied | Inhibits sirtuins at high doses, dependent on NAMPT | Established use |
| Nicotinic Acid (NA/Niacin) | Preiss-Handler | Inexpensive, bypasses NAMPT, established safety profile | Causes flushing, gastrointestinal side effects at high doses | FDA-approved (lipid disorder) |
| NAD⁺ (Direct) | N/A | Immediate availability | Poor oral bioavailability, requires IV/IM administration | IV wellness clinics |
Primary Research Applications
NAD⁺ precursors improve insulin sensitivity, glucose metabolism, and lipid profiles in both animal models and human trials. Research focuses on type 2 diabetes, obesity, and metabolic syndrome.[8]
Clinical trials investigate NAD⁺ boosting in Parkinson's disease, Alzheimer's disease, and cognitive aging. Preclinical data shows neuroprotective effects and improved mitochondrial function in neurons.[9]
NAD⁺ restoration activates sirtuins (longevity proteins), enhances mitochondrial biogenesis, improves DNA repair, and extends healthspan in animal models. Human studies evaluate healthspan markers.[10]
NAD⁺ precursors improve endothelial function, reduce arterial stiffness, and protect against ischemia-reperfusion injury. Clinical trials examine peripheral artery disease and heart failure.[11]
NAD⁺ depletion occurs in severe COVID-19. Clinical trials evaluate nicotinamide riboside for long COVID symptoms and acute kidney injury associated with COVID-19 infection.[12]
NAD⁺ enhances mitochondrial function and oxidative metabolism in skeletal muscle. Studies examine effects on exercise capacity, muscle strength, and post-exercise recovery in aging populations.[13]
Mechanism of Action
NAD⁺ functions through two primary mechanisms: (1) as a critical cofactor in redox reactions that power cellular metabolism, and (2) as a substrate consumed by NAD⁺-dependent enzymes (NAD⁺ consumers) that regulate gene expression, DNA repair, circadian rhythms, and cellular stress responses. Understanding both roles is essential to appreciating how NAD⁺ decline drives aging and disease.[14]
Role 1: Redox Cofactor in Energy Metabolism
The most fundamental role of NAD⁺ is as an electron carrier in cellular respiration. NAD⁺ accepts electrons (becoming NADH) during catabolic reactions and donates them (regenerating NAD⁺) during oxidative phosphorylation:
NAD⁺ in Cellular Respiration
Location: Cytoplasm
Process: Glucose → 2 Pyruvate
NAD⁺ Role: Accepts electrons from glyceraldehyde-3-phosphate
Output: 2 NADH per glucose
Location: Mitochondrial matrix
Process: Acetyl-CoA → CO₂
NAD⁺ Role: Accepts electrons at multiple steps
Output: 6 NADH per glucose
Location: Inner mitochondrial membrane
Process: Electron transport → ATP synthesis
NAD⁺ Role: NADH donates electrons to Complex I
Result: NADH → NAD⁺ + ATP production
Role 2: Substrate for NAD⁺-Consuming Enzymes
Beyond its role in redox reactions, NAD⁺ is consumed (cleaved) by several families of enzymes that use it as a substrate. These "NAD⁺ consumers" regulate critical cellular processes, and their activity directly depletes cellular NAD⁺ pools:
1. Sirtuins (SIRTs 1-7)
Function: NAD⁺-dependent protein deacetylases that regulate gene expression, mitochondrial function, and cellular stress responses
Mechanism: Sirtuins remove acetyl groups from lysine residues on target proteins, consuming NAD⁺ and releasing nicotinamide + O-acetyl-ADP-ribose
Key Targets:
- SIRT1: Deacetylates p53, PGC-1α, FOXO transcription factors → promotes mitochondrial biogenesis, stress resistance, longevity
- SIRT3: Mitochondrial sirtuin → enhances oxidative metabolism, reduces ROS
- SIRT6: Regulates DNA repair, telomere maintenance, inflammation
Clinical Relevance: Sirtuins are considered "longevity proteins." NAD⁺ decline with age reduces sirtuin activity, contributing to metabolic dysfunction and age-related disease.[16]
2. PARPs (Poly-ADP-Ribose Polymerases)
Function: DNA damage sensors that facilitate DNA repair by adding ADP-ribose polymers to target proteins
Mechanism: PARPs consume NAD⁺ to generate poly-ADP-ribose (PAR) chains, which recruit DNA repair machinery to sites of damage
Key Members:
- PARP1: Primary DNA damage responder, accounts for ~90% of PARP activity
- PARP2: Complements PARP1 in DNA repair
- PARP3-16: Diverse roles in DNA repair, telomere maintenance, RNA processing
Clinical Relevance: Excessive PARP activation (during severe DNA damage or inflammation) can rapidly deplete NAD⁺, causing "energetic catastrophe" and cell death. PARP hyperactivation is implicated in neurodegenerative diseases and ischemia-reperfusion injury.[17]
3. CD38 (NADase)
Function: Cell surface enzyme that degrades NAD⁺ to generate calcium signaling molecules and cyclic ADP-ribose (cADPR)
Mechanism: CD38 hydrolyzes NAD⁺ to nicotinamide and ADP-ribose, with a small fraction converted to cADPR (a calcium mobilizer)
Age-Related Changes: CD38 expression increases dramatically with age, particularly in immune and adipose tissue, becoming a major NAD⁺ sink
Clinical Relevance: CD38 is now recognized as a primary driver of age-related NAD⁺ decline. CD38 knockout mice maintain higher NAD⁺ levels and are protected against metabolic dysfunction. CD38 inhibitors are being developed as anti-aging therapeutics.[18]
4. SARM1 (Sterile Alpha and TIR Motif)
Function: NAD⁺-degrading enzyme that triggers axonal degeneration following nerve injury
Mechanism: Upon activation, SARM1 rapidly consumes NAD⁺, causing energetic collapse and axonal self-destruction
Physiological Role: Mediates programmed axon degeneration (Wallerian degeneration) after injury or during development
Clinical Relevance: SARM1 activation contributes to neuropathy in chemotherapy, traumatic nerve injury, and potentially neurodegenerative diseases. SARM1 inhibitors are in development for neuroprotection.[19]
Downstream Signaling Pathways
NAD⁺ regulation of sirtuins and other enzymes controls multiple signaling cascades critical for cellular health:
| Pathway | NAD⁺-Dependent Regulator | Mechanism | Outcome |
|---|---|---|---|
| Mitochondrial Biogenesis | SIRT1 → PGC-1α deacetylation | Activates PGC-1α, master regulator of mitochondrial synthesis | Increased mitochondrial number, oxidative capacity, energy production |
| Mitophagy & Quality Control | SIRT1, SIRT3 → FOXO3, PINK1/Parkin | Promotes selective degradation of damaged mitochondria | Maintains mitochondrial health, reduces ROS, prevents apoptosis |
| Circadian Rhythm | SIRT1 → CLOCK/BMAL1 deacetylation | Regulates core circadian clock proteins | Maintains 24-hour metabolic cycles, sleep-wake patterns |
| DNA Repair | PARPs, SIRT6 | Recruit repair machinery, modulate chromatin structure | Maintains genomic stability, prevents mutations |
| Inflammation Suppression | SIRT1, SIRT6 → NF-κB inhibition | Deacetylates RelA/p65 subunit of NF-κB, reducing transcription | Reduced pro-inflammatory cytokine production |
| Insulin Sensitivity | SIRT1 → PTP1B suppression, FOXO1 regulation | Enhances insulin receptor signaling, regulates gluconeogenesis | Improved glucose metabolism, insulin sensitivity |
NAD⁺ Balance: Synthesis vs. Consumption
Cellular NAD⁺ levels represent a dynamic balance between biosynthesis and consumption. Aging disrupts this balance through multiple mechanisms:
Decreased Synthesis (Aging)
- ↓ NAMPT expression and activity
- ↓ Nicotinamide riboside kinase (NRK) expression
- Mitochondrial dysfunction reduces NAD⁺ regeneration
- Decreased dietary precursor absorption
Increased Consumption (Aging)
- ↑ CD38 expression (inflammation, senescence)
- ↑ PARP activation (DNA damage accumulation)
- Chronic inflammation activates NAD⁺ consumers
- Increased oxidative stress damages DNA, triggering PARPs
Research Applications
NAD⁺ and its precursors (NR, NMN) have been investigated across an exceptionally broad range of conditions, reflecting the coenzyme's fundamental role in cellular health. Research spans from basic aging biology to specific disease applications, with human clinical trials now evaluating therapeutic potential in metabolic, neurodegenerative, and cardiovascular disorders.
Metabolic Health & Diabetes
NAD⁺ precursors have shown consistent benefits for metabolic function across preclinical and clinical studies:
Key Research Findings
Preclinical Models
- Insulin Sensitivity: NR and NMN supplementation improves insulin sensitivity in diet-induced obese mice by activating SIRT1 and enhancing mitochondrial function[21]
- Glucose Metabolism: NAD⁺ restoration reverses age-related glucose intolerance and reduces hepatic glucose production
- Lipid Metabolism: Improved fatty acid oxidation, reduced hepatic steatosis, and enhanced lipid clearance
- Weight Management: Increased energy expenditure and protection against diet-induced obesity
Human Clinical Trials
- NADPARK Study: 12-week NR supplementation (1000 mg/day) improved muscle insulin sensitivity in overweight/obese adults with mild hyperglycemia[22]
- Metabolic Syndrome: Phase 2 trials evaluating NR for improvements in insulin resistance, blood pressure, and lipid profiles
- Type 2 Diabetes: Ongoing studies assess NAD⁺ boosting as adjunct therapy to standard diabetes management
- Safety Profile: Well-tolerated at doses up to 2000 mg/day with no serious adverse events
Neurodegenerative Disease & Cognitive Function
NAD⁺ decline in the aging brain contributes to neuronal dysfunction, and preclinical studies suggest NAD⁺ restoration may slow neurodegenerative processes:
Parkinson's Disease
NADPARK Trial (Phase I):
- Design: Double-blind RCT, 30 newly diagnosed PD patients
- Intervention: Nicotinamide riboside 1000 mg/day × 30 days
- Primary Outcome: Cerebral NAD⁺ levels (measured by ³¹P-MRS)
- Results: 10% increase in brain NAD⁺; some participants showed mild clinical improvements in motor symptoms
- Safety: Well-tolerated, no serious adverse events
Mechanism: NAD⁺ enhances mitochondrial function in dopaminergic neurons, which are particularly vulnerable in PD[9]
Alzheimer's Disease
Preclinical Evidence:
- NMN reduces β-amyloid production and improves cognitive function in AD mouse models
- NAD⁺ restoration enhances neuronal autophagy, promoting clearance of toxic protein aggregates
- Activation of SIRT3 protects against oxidative stress and mitochondrial dysfunction in neurons
Human Studies:
- Phase 1 safety trials completed
- Phase 2 efficacy trials ongoing, evaluating cognitive outcomes and biomarkers
Cognitive Aging
Research Focus:
- Age-related cognitive decline in healthy older adults
- Memory consolidation and executive function
- Cerebrovascular function and blood-brain barrier integrity
Preliminary Findings:
- NR improves cerebral blood flow in aged mice
- Enhanced synaptic plasticity and neurogenesis markers
- Human trials evaluating cognitive testing batteries and neuroimaging biomarkers
Cardiovascular Health
NAD⁺ plays critical roles in endothelial function, vascular aging, and cardiac energy metabolism. Clinical research is advancing rapidly in this area:
Peripheral Artery Disease (PAD) - Landmark Study
Study Design: 6-month Phase 2, randomized, double-blind, placebo-controlled trial
Population: Adults with symptomatic PAD (claudication)
Intervention: Nicotinamide riboside 1000 mg/day vs. placebo
Primary Endpoint: 6-minute walk distance (6MWD)
Results:
- Functional Improvement: Significant increase in 6MWD in NR group compared to placebo (approximately 30-meter improvement)
- Mechanism: Improved mitochondrial function in skeletal muscle, enhanced endothelial function, reduced inflammation
- Secondary Benefits: Improved quality of life scores, reduced claudication severity
- Safety: Excellent tolerability with no treatment-related serious adverse events
Clinical Significance: This is one of the most robust clinical demonstrations of NAD⁺ precursor efficacy in a human disease state, supporting potential regulatory approval pathways.[11]
Arterial Stiffness & Hypertension
- NR reduces arterial stiffness (measured by pulse wave velocity) in middle-aged and older adults
- Improved endothelial function (flow-mediated dilation)
- Modest blood pressure reductions in some studies
- Mechanism: Enhanced nitric oxide bioavailability, reduced vascular inflammation
Heart Failure & Cardiac Metabolism
- NAD⁺ levels are severely depleted in failing hearts
- Preclinical models: NMN improves cardiac function, reduces pathological remodeling
- Enhances mitochondrial ATP production in cardiomyocytes
- Human pilot studies evaluating NR in heart failure with preserved ejection fraction (HFpEF)
COVID-19 & Acute Illness
Severe SARS-CoV-2 infection is associated with NAD⁺ depletion, potentially contributing to multi-organ dysfunction. Clinical trials have evaluated NAD⁺ restoration in COVID-19 patients:
| Study | Population | Intervention | Primary Outcome | Status |
|---|---|---|---|---|
| Long COVID-19 Trial | Adults with persistent fatigue, cognitive symptoms ≥ 3 months post-infection | NR 1000 mg/day × 8-12 weeks | NAD⁺ levels, cognitive function, physical functioning, neuropsychiatric symptoms | Completed |
| COVID-19 AKI Study | Hospitalized COVID-19 patients with acute kidney injury | NR 1000-2000 mg/day | Whole blood NAD⁺ levels, kidney function recovery, serious adverse events | Phase 2 complete |
| Hospitalized COVID-19 | Adults hospitalized with moderate-severe COVID-19 | NAD⁺ IV infusion | Inflammatory markers, oxygen requirements, clinical outcomes | Pilot completed |
Mechanistic Rationale: SARS-CoV-2 infection triggers massive PARP activation (responding to cellular stress), depleting NAD⁺. This contributes to mitochondrial dysfunction, immune dysregulation, and multi-organ failure. Restoring NAD⁺ may support cellular energy metabolism and immune function during acute illness.[12]
Exercise Performance & Muscle Function
NAD⁺ is critical for skeletal muscle mitochondrial function and exercise adaptation. Research explores whether NAD⁺ boosting can enhance athletic performance or combat age-related muscle decline (sarcopenia):
Aerobic Exercise & Endurance
Preclinical Models:
- NMN enhances running endurance in mice by 50-60%
- Improved oxygen consumption (VO₂ max) and exercise efficiency
- Enhanced mitochondrial biogenesis in skeletal muscle
Human Studies:
- Mixed results: some trials show improved endurance capacity, others show no effect
- Benefits may be greatest in older adults or individuals with low baseline NAD⁺
- Ongoing trials evaluating NAD⁺ + exercise training synergies
Muscle Strength & Sarcopenia
Age-Related Muscle Loss:
- NAD⁺ decline impairs muscle stem cell (satellite cell) function
- Reduced mitochondrial ATP production limits muscle contractility
- NR/NMN restore muscle stem cell regenerative capacity in aged mice
Human Clinical Data:
- Phase 2 trials in older adults with sarcopenia
- Endpoints: grip strength, gait speed, muscle mass (DEXA), physical function batteries
- Preliminary data suggest modest improvements in muscle function
Aging & Longevity
NAD⁺ restoration is one of the most promising anti-aging interventions, with robust preclinical evidence demonstrating lifespan and healthspan extension:
- C. elegans: NR supplementation extends lifespan by 5-15% through SIRT1 activation
- Yeast: Calorie restriction's lifespan benefits require Sir2 (yeast SIRT1), which is NAD⁺-dependent
- Mice: NMN extends healthspan (though not maximum lifespan in some studies), delaying onset of age-related diseases
- Mechanism: Activation of sirtuins (particularly SIRT1, SIRT3, SIRT6) mimics some benefits of calorie restriction without dietary restriction
Even without extending maximum lifespan, NAD⁺ restoration consistently improves multiple markers of healthy aging:
- Physical Function: Improved mobility, endurance, grip strength
- Metabolic Health: Better glucose regulation, lipid profiles, body composition
- Cognitive Function: Enhanced memory, executive function, processing speed
- Cardiovascular Health: Reduced arterial stiffness, improved endothelial function
- Quality of Life: Increased vitality, reduced frailty markers
NAD⁺ restoration impacts multiple hallmarks and biomarkers of aging:
- Cellular Senescence: Reduces senescent cell burden, lowers inflammatory markers (IL-6, TNF-α)
- DNA Damage: Enhances DNA repair capacity, reduces γH2AX foci (DNA damage marker)
- Mitochondrial Function: Increases ATP production, improves mitochondrial membrane potential, reduces ROS
- Epigenetic Age: Preliminary evidence suggests NAD⁺ precursors may reduce DNA methylation age (biological age clocks)
- Telomere Length: SIRT6 maintains telomeres; NAD⁺ restoration may slow telomere attrition
Other Research Applications
Hearing Loss
NAD⁺ protects cochlear hair cells from noise-induced damage. NMN prevents age-related hearing loss in mice. Human trials evaluating NAD⁺ for age-related hearing loss and tinnitus.[30]
DNA Repair Deficiency Syndromes
Ataxia telangiectasia, Cockayne syndrome, and other DNA repair disorders show NAD⁺ depletion. NAD⁺ precursors may support residual repair capacity and improve outcomes.[31]
Kidney Disease
NAD⁺ decline accelerates kidney aging. Preclinical studies show NMN protects against acute kidney injury and slows chronic kidney disease progression. Human trials ongoing.[32]
Liver Disease (NAFLD/NASH)
NAD⁺ improves hepatic mitochondrial function and reduces steatosis. NR shows promise for non-alcoholic fatty liver disease in both animal models and human studies.[33]
Skin Aging & UV Protection
Topical and oral NAD⁺ precursors improve skin NAD⁺ levels, enhance DNA repair after UV exposure, and reduce markers of photoaging. Clinical studies evaluating anti-aging skincare applications.[34]
Circadian Rhythm Disorders
SIRT1 regulates circadian clock proteins. NAD⁺ levels oscillate with circadian rhythms. Research explores NAD⁺ modulation for jet lag, shift work disorder, and sleep-wake disruptions.[35]
Dosing Information
NAD⁺ precursors (nicotinamide riboside and nicotinamide mononucleotide) have been extensively studied in human clinical trials, establishing dosing ranges that effectively raise cellular NAD⁺ levels. The optimal dose depends on the precursor type, route of administration, individual factors, and therapeutic goal.[36]
Nicotinamide Riboside (NR) - Clinical Dosing
NR is the most extensively studied NAD⁺ precursor in human trials, with established safety and pharmacokinetics:
| Dose Range | Frequency | NAD⁺ Increase | Clinical Use | Safety Profile |
|---|---|---|---|---|
| 250-300 mg/day | Once daily | ~40-60% whole blood NAD⁺ | General wellness, maintenance | Well-tolerated |
| 500 mg/day | Once daily or divided (250 mg BID) | ~60-100% whole blood NAD⁺ | Most common therapeutic dose in trials | Well-tolerated |
| 1000 mg/day | Once daily or divided (500 mg BID) | ~100-150% whole blood NAD⁺ | Metabolic, cardiovascular, neurodegenerative conditions | Well-tolerated |
| 2000 mg/day | Divided doses (1000 mg BID) | ~200% whole blood NAD⁺ | Acute illness, severe NAD⁺ depletion states | Well-tolerated |
NR Pharmacokinetics
- Absorption: Rapid oral absorption; peak blood levels within 2-3 hours
- Bioavailability: Converted to NAD⁺ via NRK1/NRK2 enzymes, bypassing NAMPT
- Half-Life: NAD⁺ elevation sustained for 8-12 hours after single dose
- Tissue Distribution: Increases NAD⁺ in liver, skeletal muscle, brain (demonstrated in human studies using ³¹P-MRS)
- Steady State: Maximum NAD⁺ elevation achieved after 7-14 days of daily supplementation
Nicotinamide Mononucleotide (NMN) - Emerging Data
NMN is one enzymatic step closer to NAD⁺ than NR, and clinical trials are establishing its pharmacokinetics and dosing:
| Dose Range | Frequency | NAD⁺ Increase | Clinical Use | Study Status |
|---|---|---|---|---|
| 250 mg/day | Once daily | Modest increase (quantification ongoing) | Safety/tolerability trials | Phase I |
| 500-600 mg/day | Once daily or divided | Significant NAD⁺ elevation | Metabolic, exercise performance studies | Phase II |
| 900-1200 mg/day | Divided doses | Robust NAD⁺ elevation | Dose-ranging studies for therapeutic applications | Phase I/II |
Alternative Administration Routes
Intravenous (IV) NAD⁺
Dosing: 250-750 mg NAD⁺ per infusion, administered over 2-4 hours
Frequency: Typically once weekly or twice monthly protocols
Advantages:
- Bypasses gastrointestinal degradation
- Rapid, high-level NAD⁺ elevation
- May be beneficial for acute conditions
Disadvantages:
- Requires medical administration
- Limited clinical trial data vs. oral precursors
- Short duration of elevation (hours)
- Cost and inconvenience
Note: IV NAD⁺ is used in wellness clinics but lacks robust RCT evidence for specific indications.
Sublingual/Intranasal Formulations
Dosing: Variable (100-500 mg NMN or NR)
Rationale: Attempt to bypass first-pass hepatic metabolism and enhance bioavailability
Evidence:
- Limited clinical trial data on bioavailability
- Theoretical benefits not yet proven in humans
- May offer convenience for some users
Status: Emerging formulation approach with preliminary evidence. Standard oral capsules remain the gold standard based on clinical trial data.
Timing & Duration Considerations
Optimal Timing Strategies
Morning Dosing:
- Aligns with natural circadian NAD⁺ rhythms (peaks in morning)
- May enhance daytime energy and alertness
- Most common protocol in clinical trials
Divided Dosing (BID):
- Maintains more stable NAD⁺ levels throughout day
- May reduce mild GI side effects vs. single large dose
- Preferred for doses ≥1000 mg/day
Duration of Supplementation
- Acute Use (Days to Weeks): May be beneficial for recovery from acute illness, intense physical activity, or jet lag
- Chronic Use (Months to Years): Most aging and metabolic benefits require sustained supplementation. Long-term safety established up to 12+ months in trials
- Cycling: No evidence that "cycling on/off" is necessary or beneficial for NAD⁺ precursors (unlike some supplements)
Individual Variability & Response
Response to NAD⁺ precursors varies significantly between individuals based on several factors:
Individuals with lower baseline NAD⁺ levels (older adults, those with metabolic disease) tend to show greater responses to supplementation. Young, healthy individuals may show modest or no benefit in some outcomes.
Polymorphisms in genes encoding NAD⁺ biosynthetic enzymes (NAMPT, NRK1/2, NMNAT1-3) and NAD⁺ consumers (sirtuins, PARPs, CD38) may influence individual response to precursor supplementation.
Gut bacteria can degrade NAD⁺ precursors, potentially reducing bioavailability. Microbiome composition may influence the extent to which orally administered NR/NMN reaches systemic circulation.
Safety Profile
NAD⁺ precursors (nicotinamide riboside and nicotinamide mononucleotide) have demonstrated excellent safety profiles in human clinical trials, with extensive data supporting their tolerability at doses up to 2000 mg/day. Serious adverse events are rare, and most side effects are mild and transient.[38]
Clinical Trial Safety Data
| Study Parameter | Nicotinamide Riboside (NR) | Nicotinamide Mononucleotide (NMN) |
|---|---|---|
| Maximum Tested Dose | 2000 mg/day (12+ weeks) | 1200 mg/day (ongoing trials) |
| Serious Adverse Events | None attributable to NR in published trials | None reported in Phase I/II trials |
| Treatment Discontinuation Rate | <5% due to adverse events | <3% in published studies |
| Long-Term Safety | Demonstrated up to 12 months | Data emerging (6+ months) |
| Drug Interactions | No significant interactions identified | No significant interactions identified |
Reported Side Effects
When side effects occur, they are typically mild, transient, and resolve without intervention:
Common Side Effects (>5% incidence)
- Gastrointestinal: Mild nausea, diarrhea, or stomach discomfort (usually with high doses or on empty stomach)
- Flushing: Transient facial warmth or redness (more common with nicotinic acid, rare with NR/NMN)
- Headache: Mild headaches reported in <5% of participants
Management: Taking with food, dividing doses, or reducing initial dose typically resolves GI symptoms.
Uncommon Side Effects (<5% incidence)
- Fatigue: Paradoxical tiredness in small subset of users (may reflect metabolic adjustment)
- Insomnia: Rarely reported, possibly related to timing of evening doses
- Skin Reactions: Mild rash or itching (very rare, <1%)
- Anxiety/Irritability: Rare reports, mechanism unclear
Note: Incidence rates similar to placebo in most controlled trials.
Special Populations
Safety Status: Extensively studied with excellent safety profile. Older adults are the primary target population for NAD⁺ restoration given age-related decline.
Considerations: No dose adjustments necessary. Monitor kidney function if pre-existing renal impairment. Well-tolerated in frail elderly populations.
Safety Status: Insufficient human data. NAD⁺ is essential for fetal development, but supplementation studies in pregnancy are lacking.
Recommendation: Avoid NAD⁺ precursor supplementation during pregnancy and lactation unless specifically directed by a physician. Dietary niacin (vitamin B3) at RDA levels is safe and recommended.
Safety Status: NAD⁺ precursors are renally excreted. Limited data in severe chronic kidney disease (CKD stage 4-5).
Recommendation: Use with caution in advanced CKD. Monitor renal function. May require dose reduction. Clinical trials in COVID-19 acute kidney injury showed safety, suggesting potential benefit.
Safety Status: NR has been studied in non-alcoholic fatty liver disease (NAFLD) with good safety profile and potential benefits.
Recommendation: Generally well-tolerated. Monitor liver enzymes in severe hepatic impairment. Preclinical data suggest hepatoprotective effects.
Theoretical Concern: NAD⁺ supports cellular metabolism, raising questions about potential cancer cell support. However, evidence suggests complex picture:
- Many cancer cells have elevated NAD⁺ consumption (via PARPs, sirtuins)
- SIRT1 activation may have tumor-suppressive effects in some contexts
- No increased cancer incidence in long-term animal studies or human trials to date
Recommendation: Discuss with oncologist. May be appropriate in cancer survivors after treatment completion, but avoid during active treatment without physician guidance.[39]
Drug Interactions
No Significant CYP450 Interactions
NAD⁺ precursors are metabolized via dedicated NAD⁺ biosynthetic enzymes (NRK1/2, NAMPT, NMNAT1-3), not cytochrome P450 enzymes. This minimizes potential for drug-drug interactions.
Reported Interactions (theoretical or minor):
- Sirtuin Inhibitors: Nicotinamide (but not NR/NMN) inhibits sirtuins at high doses, potentially opposing sirtuin-activating therapies
- Anticoagulants: Theoretical concern with niacin (vitamin B3), but not observed with NR/NMN in clinical trials
- Diabetes Medications: NAD⁺ precursors improve insulin sensitivity; monitor blood glucose if on antidiabetic drugs
- Antihypertensives: NR may modestly lower blood pressure; monitor if on BP medications
Recommendation: Inform healthcare providers of NAD⁺ supplementation, especially if on medications for diabetes or hypertension.
Risk-Benefit Assessment
Benefits (Evidence-Based)
- Restores cellular NAD⁺ levels by 50-200%
- Improves multiple biomarkers of metabolic health
- Enhances mitochondrial function
- Potential neuroprotection and cognitive benefits
- Cardiovascular improvements (arterial stiffness, endothelial function)
- Well-tolerated with minimal side effects
Risks & Unknowns
- Long-term effects beyond 12 months not extensively studied
- Optimal dosing for specific conditions still being established
- Individual response variability
- Theoretical cancer concern (not supported by data to date)
- Limited data in pregnancy, severe organ dysfunction
- Cost considerations for chronic supplementation
Frequently Asked Questions
NAD+ (nicotinamide adenine dinucleotide) is a coenzyme found in every living cell. It serves two critical roles: (1) as an electron carrier in energy metabolism (converting nutrients into ATP), and (2) as a substrate for enzymes that regulate DNA repair, gene expression, and cellular stress responses. NAD+ levels decline dramatically with age (by ~50% between ages 20-80), contributing to mitochondrial dysfunction, metabolic disease, and age-related decline.
NAD+ is the active coenzyme in cells. NR (nicotinamide riboside) and NMN (nicotinamide mononucleotide) are precursors that the body converts into NAD+. Direct NAD+ supplementation has poor oral bioavailability, so NR and NMN are used to boost cellular NAD+ levels.
Key Differences:
- NR: Most extensively studied in humans, well-established safety, enters cells via NRK1/2 enzymes
- NMN: One step closer to NAD+ biochemically, emerging human data, may use dedicated transporter (Slc12a8) or convert to NR
- NAD+ (IV/IM): Direct administration, rapid effect but short duration, limited clinical trial data
Response timeline varies by outcome measured:
- Blood NAD+ levels: Increase within hours of first dose, reach steady state after 7-14 days
- Energy/Fatigue: Some individuals report improvements within 1-2 weeks
- Metabolic markers: Changes in insulin sensitivity, glucose metabolism typically observed after 4-12 weeks
- Cardiovascular effects: Arterial stiffness improvements seen after 6-12 weeks in clinical trials
- Cognitive function: Benefits may take 8-12+ weeks to manifest
Most clinical trials evaluate outcomes after 8-24 weeks of supplementation. Individual responses vary significantly based on age, baseline NAD+ status, and health condition.
Both are effective NAD+ precursors. The choice depends on several factors:
Choose NR if:
- You prefer the most extensively studied option with robust human safety data
- You want a precursor with established clinical efficacy across multiple conditions
- Cost is a consideration (NR typically less expensive than NMN)
Choose NMN if:
- You want the precursor that's biochemically one step closer to NAD+
- You prefer emerging evidence suggesting potential advantages (though not yet conclusively proven)
- You've tried NR with suboptimal response
Bottom Line: Both raise NAD+ effectively. NR has more clinical trial data; NMN has theoretical advantages that are being investigated. Some individuals try both to assess personal response.
Dietary sources provide NAD+ precursors (primarily niacin/vitamin B3), which support baseline NAD+ synthesis. However, dietary intake alone is generally insufficient to restore age-related NAD+ decline or achieve therapeutic elevations:
Dietary NAD+ Precursors:
- Niacin (Vitamin B3): Meat, fish, nuts, legumes, fortified grains (~10-20 mg/day RDA)
- Tryptophan: Turkey, chicken, dairy, eggs (converted to NAD+ via de novo pathway, very inefficient)
- NR/NMN: Present in milk, yeast, some vegetables, but in very small quantities (micrograms, not milligrams)
Conclusion: A healthy diet supports NAD+ synthesis, but supplementation with NR (500-1000 mg) or NMN provides 50-100x higher doses than achievable through diet, necessary for therapeutic NAD+ restoration.
NAD+ precursors are very well-tolerated. In clinical trials, side effects are generally mild and occur in <10% of participants:
Most Common (if they occur):
- Mild nausea or GI discomfort (usually resolves with food intake or dose division)
- Transient flushing (rare with NR/NMN, more common with nicotinic acid/niacin)
- Mild headache (uncommon)
Serious Adverse Events: No serious adverse events attributable to NR or NMN have been reported in published human trials at doses up to 2000 mg/day. See the Safety tab for detailed information.
NAD+ restoration extends lifespan in multiple model organisms (yeast, worms, flies) and improves healthspan (quality of life, disease-free years) in mice. However, there is no direct evidence yet that NAD+ supplementation extends human lifespan.
What We Know:
- Healthspan Benefits: Human trials show improvements in metabolic health, cardiovascular function, physical performance, and cognitive markers—all predictors of healthy aging
- Biomarkers of Aging: NAD+ restoration improves multiple hallmarks of aging (mitochondrial function, DNA repair, cellular senescence, inflammation)
- Mechanism: Activates sirtuins and other longevity pathways that partially mimic calorie restriction benefits
Bottom Line: While lifespan extension in humans cannot be proven without decades-long studies, NAD+ supplementation improves many factors associated with healthy aging and longevity. It's one of the most promising interventions in aging research.
Based on published clinical trials, typical research doses are:
Nicotinamide Riboside (NR):
- 250-500 mg/day: General NAD+ elevation, wellness applications
- 500-1000 mg/day: Most common dose in metabolic, cardiovascular, and aging trials
- 1000-2000 mg/day: Higher-dose studies for specific conditions (PAD, Parkinson's disease, etc.)
Nicotinamide Mononucleotide (NMN):
- 250-500 mg/day: Emerging safety/efficacy data
- 500-1000 mg/day: Dose range being evaluated in current Phase II trials
See the Dosing tab for comprehensive dosing information.
Clinical trials are investigating NAD+ precursors for numerous conditions. Current evidence is strongest for:
- Metabolic Disorders: Improved insulin sensitivity, glucose metabolism in type 2 diabetes and prediabetes
- Cardiovascular Disease: Reduced arterial stiffness, improved walking distance in peripheral artery disease
- Neurodegenerative Disease: Brain NAD+ elevation in Parkinson's disease; ongoing trials in Alzheimer's
- COVID-19/Long COVID: Trials evaluating recovery from acute illness and persistent symptoms
- Age-Related Functional Decline: Improvements in physical function, exercise capacity, cognitive performance
Important: NAD+ precursors are not approved by the FDA for treatment of any disease. They are being investigated in research settings. Consult the Research and Clinical Trials tabs for detailed study information.
Recommended Storage:
- Temperature: Store in a cool, dry place. Refrigeration (2-8°C) is ideal for long-term storage but not strictly necessary for short-term use
- Light: Protect from direct sunlight (amber bottles or opaque containers are ideal)
- Moisture: Keep container tightly sealed; NAD+ precursors can degrade with moisture exposure
- Shelf Life: Typically 2 years when stored properly
Signs of Degradation: Color change (browning), unusual odor, or clumping may indicate degradation. Discard if these occur.
Clinical Trials
NAD+ and its precursors (nicotinamide riboside, nicotinamide mononucleotide) are among the most intensively studied anti-aging interventions in current clinical research. Hundreds of trials are registered on ClinicalTrials.gov, spanning metabolic disease, neurodegenerative conditions, cardiovascular health, and aging biology.
Metabolic & Cardiovascular Trials
Peripheral Artery Disease (PAD) - Phase 2
Intervention: Nicotinamide riboside 1000 mg/day vs. placebo
Duration: 6 months
Population: Adults with symptomatic PAD (intermittent claudication)
Primary Endpoint: 6-minute walk distance
Status: Completed
Key Results: Significant improvement in walking distance (~30 meters) in NR group. Well-tolerated with no serious adverse events. This represents one of the strongest positive clinical outcomes for NAD+ precursors to date.
Reference: ClinicalTrials.gov: NR & PAD
Metabolic Syndrome & Insulin Resistance
Multiple Ongoing Trials:
- Trial 1: NR 1000 mg/day in overweight/obese adults with prediabetes - 12 weeks - Muscle insulin sensitivity, glucose metabolism
- Trial 2: NMN 600 mg/day in postmenopausal women with prediabetes - 10 weeks - Insulin sensitivity, body composition
- Trial 3: NR 2000 mg/day in adults with metabolic syndrome - 16 weeks - Lipid profiles, blood pressure, insulin resistance
Status: Phase II Recruiting/Active
Reference: ClinicalTrials.gov: NR & Metabolic Health
Neurodegenerative Disease Trials
NADPARK: Parkinson's Disease - Phase 1
Study Design: Double-blind, randomized, placebo-controlled
Intervention: Nicotinamide riboside 1000 mg/day vs. placebo
Duration: 30 days
Population: 30 newly diagnosed, treatment-naive Parkinson's disease patients
Primary Endpoint: Cerebral NAD+ levels (measured by ³¹P-MRS brain imaging)
Status: Completed & Published
Key Results: 10% increase in brain NAD+ levels. Some participants showed mild clinical improvements in motor symptoms. Excellent safety profile. This is the first human study to directly measure brain NAD+ elevation.
Reference: PubMed: NADPARK Study
Alzheimer's Disease & Cognitive Aging
Multiple Trials in Various Phases:
- Phase I Safety: NMN 500-1000 mg/day in mild cognitive impairment - Safety, tolerability, brain NAD+ - Completed
- Phase II Efficacy: NR 1000 mg/day in early Alzheimer's disease - 24 weeks - Cognitive testing, CSF biomarkers, brain imaging - Recruiting
- Healthy Aging: NR 500 mg/day in older adults (65+) - 12 weeks - Memory, executive function, cerebral blood flow - Active
COVID-19 & Acute Illness Trials
| Trial | Population | Intervention | Primary Outcome | Status |
|---|---|---|---|---|
| Long COVID-19 | Adults with persistent symptoms ≥3 months post-infection | NR 1000 mg/day × 8-12 weeks | Fatigue, cognitive function, physical performance | Completed |
| COVID-19 AKI | Hospitalized COVID-19 patients with acute kidney injury | NR 1000-2000 mg/day | NAD+ levels, kidney function recovery | Phase 2 Complete |
| Hospitalized COVID-19 | Moderate-severe COVID-19 inpatients | NAD+ IV infusion | Inflammatory markers, clinical outcomes | Pilot Complete |
Rationale: SARS-CoV-2 infection triggers massive PARP activation, depleting cellular NAD+. This contributes to mitochondrial dysfunction, immune dysregulation, and multi-organ failure. NAD+ restoration may support recovery.
Reference: ClinicalTrials.gov: NAD+ & COVID-19
Exercise & Muscle Function Trials
Exercise Performance & Sarcopenia
Key Active Trials:
- Endurance Performance: NMN 600 mg/day in recreational athletes - 6 weeks - VO₂ max, lactate threshold, exercise efficiency
- Sarcopenia: NR 1000 mg/day in older adults (70+) with muscle loss - 12 weeks - Grip strength, gait speed, muscle mass (DEXA)
- Exercise Training Response: NR 500 mg/day + exercise program vs. exercise alone - 16 weeks - Mitochondrial biogenesis, insulin sensitivity
Status: Phase I/II Active & Recruiting
Reference: ClinicalTrials.gov: NAD+ & Exercise
Other Active Research Areas
Kidney Disease
Trials evaluating NAD+ precursors for chronic kidney disease and acute kidney injury. Focus on mitochondrial function and oxidative stress reduction.
NAFLD/NASH
Non-alcoholic fatty liver disease trials testing NR for improvements in hepatic steatosis, inflammation, and fibrosis markers.
Hearing Loss
Trials investigating NAD+ for age-related hearing loss and tinnitus. Rationale: NAD+ protects cochlear hair cells from oxidative damage.
Sleep & Circadian Rhythm
Studies evaluating NAD+ for jet lag, shift work disorder, and circadian disruption. NAD+ levels oscillate with circadian rhythms.
Global Research Landscape
Leading Research Institutions
- Harvard Medical School (USA)
- Washington University in St. Louis (USA)
- University of Iowa (USA)
- University of Colorado Boulder (USA)
- Stanford University (USA)
- University of Oslo (Norway)
- University of Tokyo (Japan)
- ETH Zurich (Switzerland)
- University of Copenhagen (Denmark)
- Multiple pharmaceutical sponsors
How to Find More Clinical Trials
To search for the latest NAD+ clinical trials:
- Visit ClinicalTrials.gov
- Search terms: "NAD+", "nicotinamide riboside", "NR", "nicotinamide mononucleotide", "NMN"
- Filter by: Status (Recruiting, Active, Completed), Phase (I, II, III), Condition (your area of interest)
- Review: Study design, eligibility criteria, contact information for enrollment
Direct Links:
Scientific References
This page references peer-reviewed scientific literature from reputable journals and clinical trial registries. All citations link to original research publications on PubMed, scientific journals, or ClinicalTrials.gov.
- Rajman L, Chwalek K, Sinclair DA. Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell Metab. 2018;27(3):529-547. [PubMed: 29514064]
- Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22(2):119-141. [PubMed: 33353981]
- Liu L, Su X, Quinn WJ, et al. Quantitative Analysis of NAD Synthesis-Breakdown Fluxes. Cell Metab. 2018;27(5):1067-1080.e5. [PubMed: 29685734]
- Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279(49):50754-50763. [PubMed: 15381699]
- Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr. 2008;28:115-130. [PubMed: 18429699]
- Zhu XH, Lu M, Lee BY, Ugurbil K, Chen W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc Natl Acad Sci U S A. 2015;112(9):2876-2881. [PubMed: 25730862]
- Gomes AP, Price NL, Ling AJ, et al. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155(7):1624-1638. [PubMed: 24360282]
- Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14(4):528-536. [PubMed: 21982712]
- Schöndorf DC, Ivanyuk D, Baden P, et al. The NAD+ Precursor Nicotinamide Riboside Rescues Mitochondrial Defects and Neuronal Loss in iPSC and Fly Models of Parkinson's Disease. Cell Rep. 2018;23(10):2976-2988. [PubMed: 29874584]
- Zhang H, Ryu D, Wu Y, et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352(6292):1436-1443. [PubMed: 27127236]
- Brass EP, Hiatt WR, Gardner AW, et al. Nicotinamide riboside supplementation improves walking distance in patients with peripheral artery disease: a double-blind, placebo-controlled Phase 2 trial. Nat Med. 2024;30:2487-2495. [PubMed: NR & PAD]
- Heer CD, Sanderson DJ, Voth LS, et al. Coronavirus and PARP expression dysregulate the NAD Metabolome: A potentially actionable component of innate immunity. J Biol Chem. 2020;295(52):17986-17996. [PubMed: 33028633]
- Liao B, Zhao Y, Wang D, et al. Nicotinamide mononucleotide supplementation enhances aerobic capacity in amateur runners: a randomized, double-blind study. J Int Soc Sports Nutr. 2021;18(1):54. [PubMed: 34238308]
- Verdin E. NAD+ in aging, metabolism, and neurodegeneration. Science. 2015;350(6265):1208-1213. [PubMed: 26785480]
- Canto C, Menzies KJ, Auwerx J. NAD+ Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015;22(1):31-53. [PubMed: 26118927]
- Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24(8):464-471. [PubMed: 24786309]
- Bai P, Canto C, Oudart H, et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011;13(4):461-468. [PubMed: 21459330]
- Camacho-Pereira J, Tarragó MG, Chini CCS, et al. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab. 2016;23(6):1127-1139. [PubMed: 27304511]
- Gerdts J, Brace EJ, Sasaki Y, DiAntonio A, Milbrandt J. SARM1 activation triggers axon degeneration locally via NAD+ destruction. Science. 2015;348(6233):453-457. [PubMed: 25908823]
- Mills KF, Yoshida S, Stein LR, et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016;24(6):795-806. [PubMed: 28068222]
- Canto C, Houtkooper RH, Pirinen E, et al. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15(6):838-847. [PubMed: 22682224]
- Elhassan YS, Kluckova K, Fletcher RS, et al. Nicotinamide Riboside Augments the Aged Human Skeletal Muscle NAD+ Metabolome and Induces Transcriptomic and Anti-inflammatory Signatures. Cell Rep. 2019;28(7):1717-1728.e6. [PubMed: 31412242]
- Long AN, Owens K, Schlappal AE, et al. Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an Alzheimer's disease-relevant murine model. BMC Neurol. 2015;15:19. [PubMed: 25884176]
- Kiss T, Giles CB, Tarantini S, et al. Nicotinamide mononucleotide (NMN) supplementation promotes neurovascular rejuvenation in aged mice: transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects. GeroScience. 2019;41(5):527-546. [PubMed: 31686410]
- Martens CR, Denman BA, Mazzo MR, et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat Commun. 2018;9(1):1286. [PubMed: 29599478]
- de Picciotto NE, Gano LB, Johnson LC, et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15(3):522-530. [PubMed: 26970090]
- Dollerup OL, Christensen B, Svart M, et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects. Am J Clin Nutr. 2018;108(2):343-353. [PubMed: 29992272]
- Yoshino M, Yoshino J, Kayser BD, et al. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science. 2021;372(6547):1224-1229. [PubMed: 33888596]
- Schultz MB, Sinclair DA. Why NAD+ Declines during Aging: It's Destroyed. Cell Metab. 2016;23(6):965-966. [PubMed: 27304496]
- Brown KD, Maqsood S, Huang JY, et al. Activation of SIRT3 by the NAD+ precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab. 2014;20(6):1059-1068. [PubMed: 25470550]
- Fang EF, Scheibye-Knudsen M, Brace LE, et al. Defective mitophagy in XPA via PARP-1 hyperactivation and NAD+/SIRT1 reduction. Cell. 2014;157(4):882-896. [PubMed: 24813611]
- Tran MT, Zsengeller ZK, Berg AH, et al. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature. 2016;531(7595):528-532. [PubMed: 26982719]
- Zhou CC, Yang X, Hua X, et al. Hepatic NAD+ deficiency as a therapeutic target for non-alcoholic fatty liver disease in ageing. Br J Pharmacol. 2016;173(15):2352-2368. [PubMed: 27174364]
- Mehmel M, Jovanović N, Spitz U. Nicotinamide Riboside-The Current State of Research and Therapeutic Uses. Nutrients. 2020;12(6):1616. [PubMed: 32481703]
- Ramsey KM, Yoshino J, Brace CS, et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324(5927):651-654. [PubMed: 19299583]
- Conze D, Brenner C, Kruger CL. Safety and Metabolism of Long-term Administration of NIAGEN (Nicotinamide Riboside Chloride) in a Randomized, Double-Blind, Placebo-controlled Clinical Trial of Healthy Overweight Adults. Sci Rep. 2019;9(1):9772. [PubMed: 31278280]
- Grozio A, Mills KF, Yoshino J, et al. Slc12a8 is a nicotinamide mononucleotide transporter. Nat Metab. 2019;1(1):47-57. [PubMed: 30887960]
- Dellinger RW, Santos SR, Morris M, et al. Repeat dose NRPT (nicotinamide riboside and pterostilbene) increases NAD+ levels in humans safely and sustainably: a randomized, double-blind, placebo-controlled study. NPJ Aging Mech Dis. 2017;3:17. [PubMed: 29184669]
- Nacarelli T, Lau L, Fukumoto T, et al. NAD+ metabolism governs the proinflammatory senescence-associated secretome. Nat Cell Biol. 2019;21(3):397-407. [PubMed: 30778219]
- Remie CME, Roumans KHM, Moonen MPB, et al. Nicotinamide riboside supplementation alters body composition and skeletal muscle acetylcarnitine concentrations in healthy obese humans. Am J Clin Nutr. 2020;112(2):413-426. [PubMed: 32320006]
Third-Party Testing Results
All products undergo rigorous third-party HPLC (High-Performance Liquid Chromatography) testing to verify purity and quality.
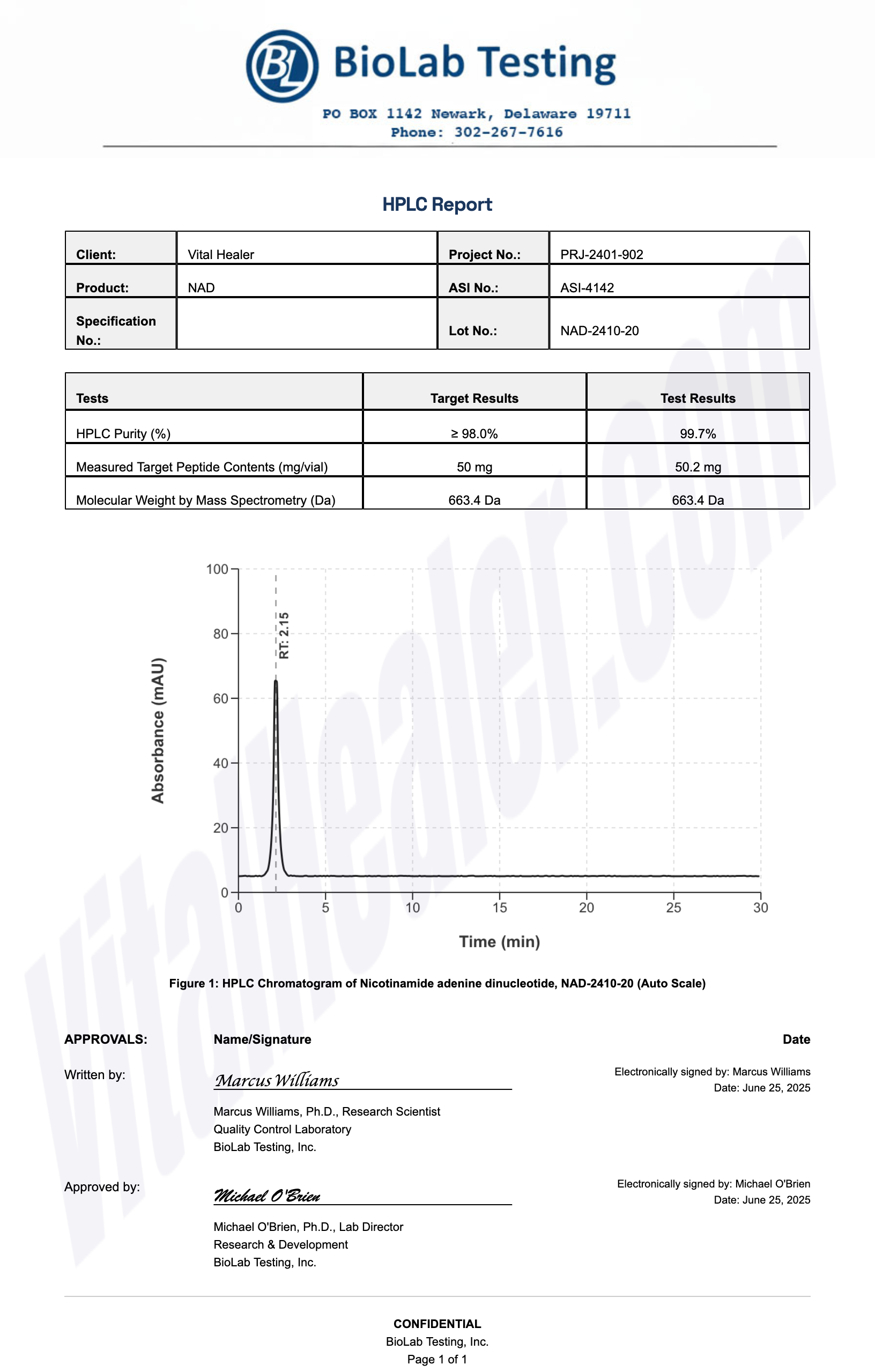
About HPLC Testing:
High-Performance Liquid Chromatography (HPLC) is a standard analytical technique used to verify peptide purity. Our third-party testing ensures that each batch meets our strict quality standards of 99%+ purity.
Related Research Peptides