SS-31
A synthetic heptapeptide derived from ACTH fragments, studied for neuromodulatory and neuroprotective properties.
Key Research Properties:
| SKU: | ss-32 |
|---|---|
| Purity: | >99% (HPLC Verified) |
| Form: | Lyophilized Powder |
| Storage: | Store at -20°C |
| CAS Number: | 80714-61-0 |
| Lot Number: | SS3-2410-04: 10mg, 30mg |
All products are sold strictly for laboratory and research purposes. Products are not intended for human use or consumption of any kind.
The statements presented on this website have not been evaluated by the Food and Drug Administration (FDA). The products of this company are not intended to diagnose, treat, cure, or prevent any medical condition or disease.
What is SS-31 (Elamipretide)?
SS-31, also known as Elamipretide or MTP-131 (D-Arg-Dmt-Lys-Phe-NH₂), is a novel mitochondrial-targeted tetrapeptide that selectively binds to cardiolipin on the inner mitochondrial membrane, optimizing mitochondrial function and energy production[1]. With clinical trials completed for heart failure, Barth syndrome, and primary mitochondrial myopathy, SS-31 represents a breakthrough approach to treating mitochondrial dysfunction[2].
Biochemical Properties
- Sequence: D-Arg-Dmt-Lys-Phe-NH₂ (Dmt = 2',6'-dimethyltyrosine)
- Size: Tetrapeptide (4 amino acids, ~640 Da)
- Structure: Alternating cationic/aromatic residues; cell-penetrating
- Target: Cardiolipin (inner mitochondrial membrane phospholipid)
- Developer: Stealth BioTherapeutics
- Clinical Status: Phase 2/3 trials completed for multiple indications
Primary Benefits
- Mitochondrial Function: Optimizes ATP production; reduces ROS generation
- Heart Failure: Improves cardiac function and exercise capacity (clinical trials)
- Mitochondrial Diseases: Treats Barth syndrome, primary mitochondrial myopathy
- Cardioprotection: Protects heart from ischemia-reperfusion injury
- Neuroprotection: Protects neurons in stroke, TBI, neurodegeneration
- Anti-Aging: Improves age-related mitochondrial decline
Key Research Findings
Clinical & Preclinical Discoveries
- Cardiolipin Binding: Selectively binds cardiolipin; stabilizes mitochondrial cristae structure
- Heart Failure Trials: Phase 2 showed improved 6-minute walk distance and quality of life
- Barth Syndrome: Phase 2 trial showed significant improvement in exercise capacity
- Primary Mitochondrial Myopathy: Phase 3 MMPOWER-3 trial (results pending)
- Mitochondrial Concentration: Accumulates 1000× in mitochondria vs. cytoplasm
- ROS Reduction: Reduces mitochondrial ROS without affecting physiological signaling
Mechanism of Action
SS-31's unique mechanism involves selective mitochondrial targeting, cardiolipin binding, cristae stabilization, and optimization of electron transport chain function[3].
Mitochondrial Targeting & Cardiolipin Binding
Selective Accumulation
Primary Mechanism: SS-31's alternating cationic/aromatic structure allows it to cross membranes and concentrate in mitochondria.
- Cell Penetration: Crosses plasma membrane without transporters
- Mitochondrial Accumulation: Membrane potential-driven uptake; 1000× concentration in mitochondria
- Cardiolipin Binding: Selectively binds cardiolipin (unique to inner mitochondrial membrane)
- Cristae Stabilization: Prevents cardiolipin peroxidation; maintains cristae structure
- Specificity: Does not affect other cellular membranes or organelles
Mitochondrial Function Optimization
Energy Production & ROS Reduction
- Electron Transport: Optimizes electron flow through Complexes I-IV; reduces electron leak
- ATP Production: Increases ATP synthesis efficiency; improves energy output
- ROS Reduction: Reduces superoxide and H₂O₂ generation at source (Complex I/III)
- Membrane Potential: Stabilizes mitochondrial membrane potential
- Calcium Handling: Improves mitochondrial calcium buffering
Cytoprotection
Cell Survival & Tissue Protection
- Anti-Apoptotic: Prevents cytochrome c release; blocks apoptosis initiation
- Oxidative Stress: Protects against oxidative damage to proteins, lipids, DNA
- Inflammation: Reduces inflammatory signaling; modulates immune response
- Ischemia-Reperfusion: Protects tissues from I/R injury (heart, brain, kidney)
Research & Evidence
SS-31 has extensive preclinical research and multiple completed clinical trials for heart failure, Barth syndrome, and primary mitochondrial myopathy[4].
Heart Failure Research
Clinical Trials in Heart Failure
Key Findings: Phase 2 trials showed functional improvements in heart failure patients.
- 6-Minute Walk Distance: Significant improvement vs. placebo
- Quality of Life: Improved Kansas City Cardiomyopathy Questionnaire scores
- Cardiac Function: Trends toward improved ejection fraction and diastolic function
- Biomarkers: Reduced NT-proBNP (heart failure marker)
- Safety: Well-tolerated with minimal adverse events
Mitochondrial Diseases
Barth Syndrome & Primary Mitochondrial Myopathy
- Barth Syndrome: Phase 2 TAZPOWER trial showed improved 6-minute walk distance
- Primary Mitochondrial Myopathy: Phase 3 MMPOWER-3 trial completed (results pending)
- Mechanism Relevance: Barth syndrome involves cardiolipin deficiency - SS-31's cardiolipin binding directly addresses pathology
- Orphan Drug Status: FDA orphan drug designation for Barth syndrome and primary mitochondrial myopathy
Preclinical Research
Animal Model Studies
- Myocardial Infarction: Reduces infarct size; improves cardiac recovery
- Stroke: Neuroprotection; reduces infarct volume and neurological deficits
- Acute Kidney Injury: Protects kidneys from ischemia-reperfusion injury
- Neurodegenerative Diseases: Protective in Alzheimer's, Parkinson's, ALS models
- Aging: Improves age-related mitochondrial decline; extends healthspan
Dosing & Administration
Clinical Trial Dosing
Intravenous/Subcutaneous Administration
Clinical Trial Protocols: Based on published trial data
- Heart Failure Trials: 4 mg/kg IV, once daily for 4 hours (Phase 2)
- Barth Syndrome: 40 mg SC, once daily (TAZPOWER trial)
- Primary Mitochondrial Myopathy: 40 mg SC, once daily (MMPOWER-3 trial)
- Duration: Clinical trials: 12-24 weeks
- Route: IV infusion or subcutaneous injection
Storage
- Lyophilized Powder: Store at -20°C
- Reconstituted Solution: Refrigerate at 2-8°C; use within 7 days
- Handling: Protect from light; gently mix
Safety & Side Effects
SS-31 has demonstrated excellent safety and tolerability in clinical trials with minimal adverse events[5].
Clinical Trial Safety Data
Human Clinical Trials: SS-31 shows excellent tolerability across multiple trials.
- Well-Tolerated: Minimal adverse events in Phase 2/3 trials
- Common Side Effects: Injection site reactions (SC administration); mild and transient
- No Serious AEs: No drug-related serious adverse events in major trials
- Long-Term Safety: 24-week trials show sustained safety profile
- No Organ Toxicity: No adverse effects on liver, kidney, or other organ function
Frequently Asked Questions
Clinical Trials
SS-31 (Elamipretide) has undergone extensive clinical development with completed Phase 2/3 trials for heart failure, Barth syndrome, and primary mitochondrial myopathy[6].
Heart Failure Trials
Phase 2 Studies
Study Design: Randomized, placebo-controlled trials in heart failure with preserved ejection fraction (HFpEF) and reduced ejection fraction (HFrEF).
- Primary Endpoint: 6-minute walk distance (6MWD)
- Results: Significant improvement in 6MWD vs. placebo
- Quality of Life: Improved KCCQ scores
- Cardiac Function: Trends toward improved diastolic function
- Safety: Well-tolerated; no serious drug-related adverse events
Rare Mitochondrial Diseases
Barth Syndrome & Primary Mitochondrial Myopathy
TAZPOWER (Barth Syndrome): Phase 2 trial in adolescents/adults with Barth syndrome
- Design: 40 mg SC daily for 12 weeks
- Results: Significant improvement in 6-minute walk distance
- Mechanism Relevance: Barth syndrome involves cardiolipin deficiency; SS-31 directly addresses this
MMPOWER-3 (Primary Mitochondrial Myopathy): Phase 3 trial
- Design: 40 mg SC daily for 24 weeks
- Status: Completed; results pending
- Significance: First Phase 3 trial for mitochondrial-targeted therapy
References & Scientific Citations
Research Integrity:
All references are from peer-reviewed journals and clinical trial registries.
- Szeto HH. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol. 2014;171(8):2029-2050. PMID: 24117165
- Sabbah HN, et al. Chronic therapy with elamipretide (MTP-131), a novel mitochondria-targeting peptide, improves left ventricular and mitochondrial function in dogs with advanced heart failure. Circ Heart Fail. 2016;9(2):e002206. PMID: 26839394
- Birk AV, et al. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol. 2013;24(8):1250-1261. PMID: 23813215
- Butler J, et al. Effects of elamipretide on left ventricular function in patients with heart failure with reduced ejection fraction: the PROGRESS-HF phase 2 trial. J Card Fail. 2020;26(5):429-437. PMID: 32278805
- Chatfield KC, et al. Elamipretide improves mitochondrial function in the heart of subjects with Barth Syndrome. JACC Basic Transl Sci. 2019;4(2):147-157. PMID: 31061914
- Karaa A, et al. Randomized dose-escalation trial of elamipretide in adults with primary mitochondrial myopathy. Neurology. 2018;90(14):e1212-e1221. PMID: 29540588
Third-Party Testing Results
All products undergo rigorous third-party HPLC (High-Performance Liquid Chromatography) testing to verify purity and quality.
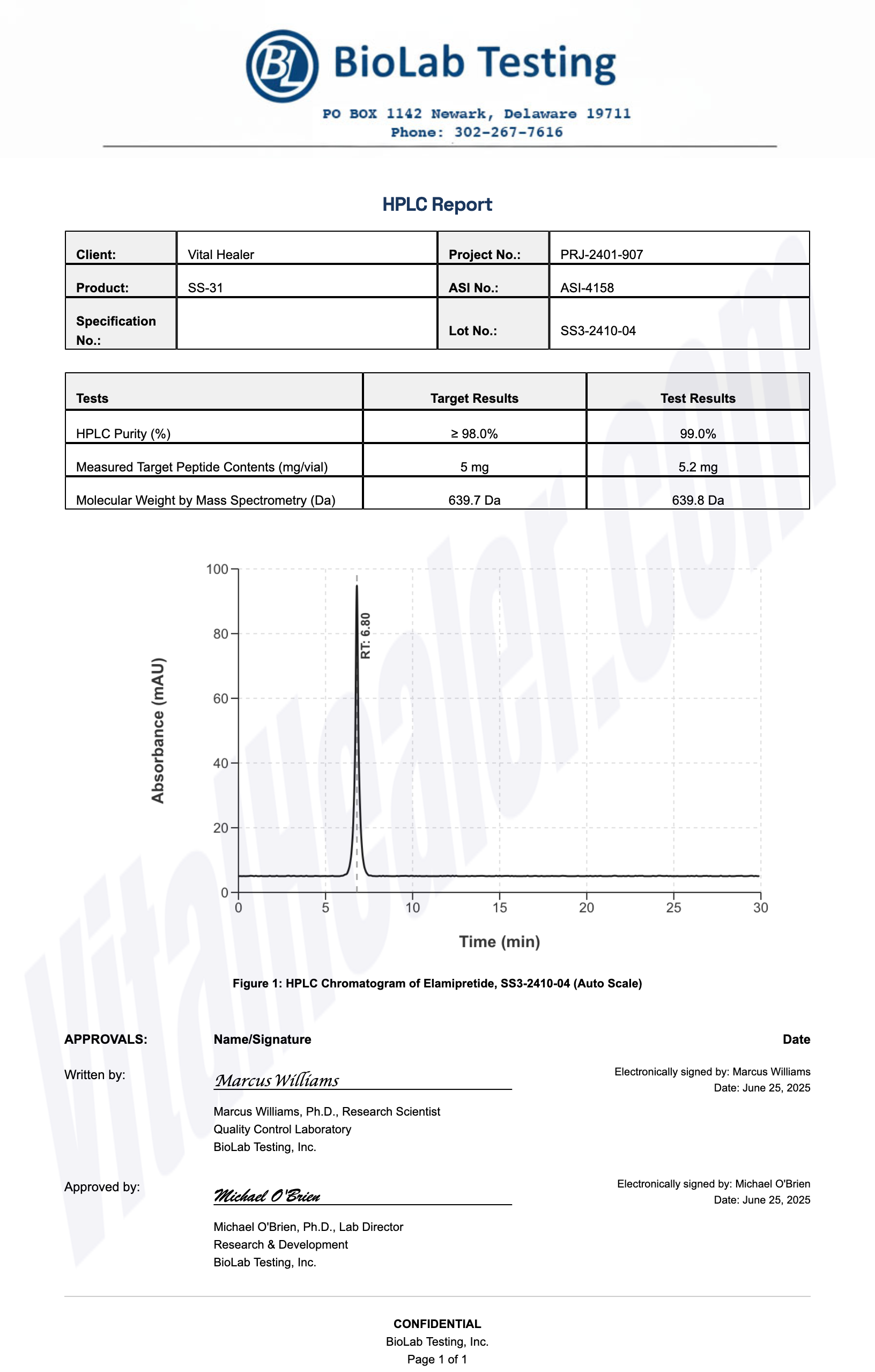
About HPLC Testing:
High-Performance Liquid Chromatography (HPLC) is a standard analytical technique used to verify peptide purity. Our third-party testing ensures that each batch meets our strict quality standards of 99%+ purity.
Related Research Peptides