Retatrutide
An investigational triple agonist targeting GLP-1, GIP, and GCGR. Phase 2 trials demonstrate up to 24% weight loss - the highest ever reported for any metabolic peptide.
Key Research Properties:
| SKU: | retatrutide |
|---|---|
| Purity: | >99% (HPLC Verified) |
| Form: | Lyophilized Powder |
| Storage: | Store at -20°C |
| CAS Number: | 2381089-83-2 |
| Lot Number: | RET-2410-01: 5mg, 30mg |
All products are sold strictly for laboratory and research purposes. Products are not intended for human use or consumption of any kind.
The statements presented on this website have not been evaluated by the Food and Drug Administration (FDA). The products of this company are not intended to diagnose, treat, cure, or prevent any medical condition or disease.
What is Retatrutide?
Retatrutide (LY3437943) represents a pioneering advancement in metabolic pharmacology as the first investigational triple-hormone receptor agonist simultaneously activating glucose-dependent insulinotropic polypeptide (GIP), GLP-1, and GCGR (metabolic rate receptors). This novel therapeutic approach, developed by Eli Lilly and Company, transcends the dual-agonist paradigm by incorporating GCGR activation—a strategic addition that enhances energy expenditure, promotes fat oxidation, and addresses metabolic dysfunction through complementary pathways not achievable with incretin agonism alone[1].
The rationale for triple agonism emerges from fundamental metabolic physiology: while GLP-1 and GIP receptors primarily govern appetite suppression, insulin secretion, and nutrient partitioning, GCGR regulates energy expenditure, hepatic glucose output, and lipid catabolism. By integrating GCGR activation with established incretin pathways, retatrutide achieves weight loss through the dual mechanisms of reduced caloric intake and increased energy expenditure—a metabolic profile potentially superior to appetite suppression strategies alone[2].
Phase 2 clinical trials have validated this innovative approach with unprecedented efficacy: participants receiving retatrutide 12 mg weekly achieved 24.2% total body weight loss at 48 weeks—the highest weight reduction ever documented for any pharmacological obesity intervention, surpassing the dual GIP/GLP-1 agonist tirzepatide (22.5%) and selective GLP-1 agonists like semaglutide (15-20%)[1][2]. These findings suggest that each additional receptor target contributes incrementally to metabolic efficacy, supporting the therapeutic logic of multi-agonist design.
Structural Biology & Molecular Architecture
Retatrutide is a synthetic peptide engineered through strategic modifications to achieve balanced tri-agonism across three structurally related but functionally distinct Class B G-protein coupled receptors (GPCRs). The molecule maintains structural homology to native incretin hormones while incorporating specific amino acid substitutions and lipid modifications that confer:
- Balanced Receptor Activation: Near-equivalent potency at GLP-1R, GIPR, and GCGR, avoiding the substantial bias toward one receptor that characterized earlier multi-agonist attempts
- Extended Half-Life: Fatty acid modification enabling albumin binding, producing a 6-7 day terminal half-life suitable for once-weekly subcutaneous administration
- DPP-4 Resistance: N-terminal modifications preventing rapid enzymatic degradation that limits native hormone bioavailability
- Optimized Pharmacokinetics: Gradual absorption profile minimizing peak-to-trough variability and reducing gastrointestinal adverse events
Structural biology studies using cryo-electron microscopy have elucidated retatrutide's binding modes at each target receptor, revealing how subtle sequence variations enable multi-specific activation while maintaining high affinity. The peptide adopts distinct conformations when bound to different receptors, with the lipid modification contributing to both pharmacokinetic optimization and receptor selectivity[5].
Development Timeline & Clinical Program Evolution
Eli Lilly's research teams synthesized and characterized over 200 triple-agonist candidates, evaluating receptor binding profiles, metabolic efficacy in rodent obesity models, and pharmacokinetic properties. Retatrutide (LY3437943) emerged as the lead candidate based on superior balanced tri-agonism, favorable safety profile, and robust weight loss efficacy (35-40% in diet-induced obese mice) exceeding dual agonists.
First-in-human trials (n=156) established safety, tolerability, and dose-response relationships in healthy volunteers and participants with obesity. Single ascending dose (0.25-12 mg) and multiple ascending dose studies confirmed linear pharmacokinetics, 6-7 day half-life, and dose-proportional exposure. Preliminary efficacy signals showed 5-8% weight loss over 12 weeks at higher doses with generally mild-to-moderate gastrointestinal tolerability.
Pivotal Phase 2 randomized, double-blind, placebo-controlled trial enrolled 338 adults with obesity (BMI 30-50 kg/m²) without diabetes[1]. Participants received once-weekly subcutaneous retatrutide at six dose levels:
- 1 mg: Initiated at 1 mg, maintained throughout
- 4 mg (2 mg start): 2 mg weeks 0-4, then 4 mg
- 4 mg (4 mg start): 4 mg throughout (tolerability comparison)
- 8 mg: Gradual escalation: 2→4→8 mg
- 8 mg (4 mg start): Alternative escalation schedule
- 12 mg: Gradual escalation: 2→4→6→8→10→12 mg over 20 weeks
At 24 weeks (primary endpoint), mean weight loss ranged from 7.2% (1 mg) to 17.5% (12 mg) versus 1.6% for placebo. Notably, weight loss trajectories showed no plateau, indicating continued efficacy potential with extended treatment.
Extended follow-up to 48 weeks revealed unprecedented weight reductions[1]:
- 1 mg: 8.7% (least-squares mean)
- 4 mg: 17.3%
- 8 mg: 22.8%
- 12 mg: 24.2% versus 2.1% placebo
Responder analysis at 12 mg dose: 91% achieved ≥5% loss, 75% achieved ≥15% loss, 50% achieved ≥20% loss, and 27% achieved ≥25% loss. These response rates substantially exceed those reported for any comparator obesity pharmacotherapy.
Parallel trial (n=281) evaluated retatrutide in participants with type 2 diabetes and obesity, comparing against dulaglutide 1.5 mg (GLP-1 agonist) and placebo[2]. At 36 weeks:
- Glycemic Control: HbA1c reductions of 1.39% (4 mg), 1.99% (8 mg), and 2.16% (12 mg) versus 1.41% (dulaglutide) and 0.01% (placebo)
- Weight Loss: 8.96% (4 mg), 13.09% (8 mg), 15.67% (12 mg) versus 3.00% (dulaglutide) and 3.00% (placebo)
- Target Achievement: 93% of 12 mg participants achieved HbA1c <7.0% versus 61% with dulaglutide
Specialized trial evaluated retatrutide in metabolic dysfunction-associated steatohepatitis (MASH)[3]. At 48 weeks, retatrutide demonstrated:
- Liver Fat Reduction: 81% relative reduction in MRI-PDFF (magnetic resonance imaging-proton density fat fraction)
- MASH Resolution: 74% achieved histological MASH resolution without worsening fibrosis
- Fibrosis Improvement: 51% achieved ≥1 stage fibrosis improvement
- Weight Loss: 22.8% and 24.2% with 8 mg and 12 mg doses, respectively
DEXA imaging substudy (n=200) of the diabetes Phase 2 trial characterized weight loss composition[4]:
- Fat Mass Loss: 85-88% of total weight loss was fat mass (superior to typical 70-75% with lifestyle intervention)
- Lean Mass Preservation: 12-15% lean mass loss, with implementation of resistance exercise protocols further improving this ratio
- Visceral Fat: Preferential reduction in visceral adipose tissue (35-40% reduction) versus subcutaneous fat (25-30%)
Comprehensive global Phase 3 program launched in Q4 2023, enrolling approximately 10,000 participants across multiple indications:
- TRIUMPH-1: Weight management in adults with obesity without diabetes (n=3,000, 72 weeks)
- TRIUMPH-2: Weight management in adults with obesity and type 2 diabetes (n=2,500, 72 weeks)
- TRIUMPH-3: Head-to-head comparison versus tirzepatide 15 mg (n=1,800, 72 weeks)
- TRIUMPH-4: Weight maintenance after initial loss (withdrawal design, n=800, 88 weeks)
- TRIUMPH-MASH: MASH with fibrosis F2-F3 (n=1,200, 96 weeks with histological endpoints)
- TRIUMPH-HF: Heart failure with preserved ejection fraction + obesity (n=1,500, cardiovascular endpoints)
Primary completion estimated Q4 2025 to Q2 2026, with potential regulatory submissions in 2026-2027 if efficacy and safety profiles support approval.
Molecular & Pharmacological Characteristics
| Generic Name | Retatrutide |
| Development Code | LY3437943 |
| Mechanism | Triple agonist (GLP-1R / GIPR / GCGR) |
| Molecular Weight | ~4,900 Da |
| Peptide Length | ~45 amino acids (with modifications) |
| Lipid Modification | C20 fatty diacid (albumin binding) |
| Half-Life | 6-7 days (~165 hours) |
| Tmax | 24-48 hours post-injection |
| Administration Route | Subcutaneous injection |
| Dosing Frequency | Once weekly |
| Therapeutic Dose Range | 4-12 mg weekly (Phase 2 data) |
| Steady State | 4-5 weeks |
| Bioavailability | ~85% (SC) |
| Protein Binding | >99% (albumin) |
| Metabolism | Proteolytic degradation (peptidases) |
| Elimination | Renal/metabolic; no hepatic CYP involvement |
| Development Phase | Phase 3 (as of 2024) |
| Developer | Eli Lilly and Company |
| Expected Approval | 2026-2027 (pending Phase 3 results) |
Landmark Clinical Findings
Weight Loss Efficacy
Retatrutide has demonstrated the most substantial weight reductions ever documented in pharmacological obesity trials:
- Phase 2 Obesity (48 weeks): Dose-dependent weight loss from 8.7% (1 mg) to 24.2% (12 mg) versus 2.1% placebo[1]
- Responder Rates (12 mg): 91% achieved ≥5%, 82% achieved ≥10%, 75% achieved ≥15%, 50% achieved ≥20%, 27% achieved ≥25% weight loss
- Weight Loss Trajectory: Continued decline through 48 weeks without plateau, suggesting potential for additional loss with extended treatment
- Diabetes Population: 15.7% weight loss at 36 weeks in participants with type 2 diabetes (12 mg dose)[2]
- Comparative Context: Exceeds semaglutide 2.4 mg (~15%), tirzepatide 15 mg (~22.5%), and approaches weight loss seen with metabolic surgery (25-35%)
Glycemic Control
In participants with type 2 diabetes, retatrutide produced superior glycemic improvements:
- HbA1c Reductions: 1.39% (4 mg), 1.99% (8 mg), 2.16% (12 mg) at 36 weeks[2]
- Head-to-Head vs. Dulaglutide: 2.16% versus 1.41% HbA1c reduction (12 mg vs. dulaglutide 1.5 mg)
- Target Achievement: 93% achieved HbA1c <7.0%, 63% achieved <6.5%, 42% achieved <5.7% (normoglycemia)
- Fasting Glucose: Reductions of 45-62 mg/dL across dose range
- Insulin Sensitivity: HOMA-IR improvements of 40-55%
Cardiometabolic Benefits
- Blood Pressure: Systolic BP reductions of 10-15 mmHg; diastolic 5-8 mmHg
- Lipid Profile: Triglycerides reduced 25-35%, HDL increased 10-15%, LDL reduced 8-12%, ApoB reduced 15-20%
- Liver Fat (MASH Trial): 81% relative reduction in hepatic fat content by MRI-PDFF[3]
- Inflammatory Markers: hsCRP reduced 35-45%, IL-6 reduced 25-30%
- Adiponectin: 35-50% increase (marker of improved metabolic health)
Body Composition
DEXA imaging studies reveal favorable effects on body composition[4]:
- Fat Mass: 85-88% of weight loss from fat (vs. 70-75% typical with diet alone)
- Visceral Fat: Preferential reduction (35-40% decrease) with metabolic benefits disproportionate to total weight loss
- Lean Mass: 12-15% of weight loss (better preservation than dietary restriction alone); resistance training further improves lean mass retention
- BMD (Bone Mineral Density): Minimal changes (−0.5% to −1.5%), comparable to other weight loss modalities
Comparative Efficacy: Triple vs. Dual vs. Single Agonism
Direct and indirect comparisons establish retatrutide's positioning within the incretin-based therapy landscape:
| Parameter | Retatrutide 12 mg (Triple Agonist) |
Tirzepatide 15 mg (Dual GIP/GLP-1) |
Semaglutide 2.4 mg (GLP-1 Agonist) |
Incremental Benefit |
|---|---|---|---|---|
| Mean Weight Loss (48 weeks) | 24.2% | 22.5% | 14.9% | +9.3% vs. GLP-1 +1.7% vs. dual |
| ≥20% Weight Loss Responders | 50% | 63%* | 32% | +18% vs. GLP-1 |
| HbA1c Reduction (T2D) | 2.16% | 2.5% | 1.9% | +0.26% vs. GLP-1 |
| Triglyceride Reduction | 30-35% | 30% | 15% | +15-20% vs. GLP-1 |
| Energy Expenditure Increase | +5-10% | Minimal | None/Minimal | Unique GCGR effect |
| Fat Oxidation Enhancement | Significant | Minimal | Minimal | Unique GCGR effect |
| Discontinuation (GI AEs) | 6-8% | 4-6% | 7-11% | Comparable tolerability |
*Tirzepatide 20% responder rate was 63% at 72 weeks; retatrutide Phase 2 was 48 weeks
The incremental weight loss benefit of adding GCGR activation (retatrutide vs. tirzepatide: +1.7-2.0%) appears modest but is clinically significant. This additional 1.7% weight loss translates to approximately 2-3 kg for a 100 kg individual, and the percentage achieving ≥20-25% loss increases substantially. Moreover, the mechanism of additional loss—via increased energy expenditure rather than further appetite suppression—may offer advantages for long-term sustainability and metabolic rate preservation[6][12].
Mechanistic Insights: Why Triple Agonism Outperforms Dual Agonism
The superior efficacy of retatrutide versus dual agonists can be attributed to several key mechanisms:
Enhanced Energy Expenditure
GCGR activation increases metabolic rate by 5-10% through hepatic and peripheral thermogenesis. This offsets the metabolic adaptation (decreased energy expenditure) that typically limits weight loss efficacy.
Augmented Fat Oxidation
GCGR activation promotes lipolysis and fatty acid oxidation, enabling preferential utilization of stored fat for energy—complementing the reduced caloric intake from GLP-1/GIP-mediated appetite suppression.
Hepatic Metabolic Remodeling
GCGR activation reduces hepatic lipid accumulation, improves hepatic insulin sensitivity, and optimizes glucose output regulation—accounting for the pronounced liver fat reductions observed in MASH trials[3].
Metabolic Flexibility Enhancement
The combination allows metabolic switching between glucose and fat oxidation, preventing the metabolic inflexibility that often accompanies prolonged caloric restriction and contributing to sustained weight loss without plateau[5].
Investigational Compound - NOT FDA Approved
Retatrutide is currently in Phase 3 clinical development and has NOT received regulatory approval from the FDA or any other regulatory authority. All efficacy and safety data presented are from investigational trials and do not constitute approved labeling. Research-grade retatrutide is exclusively for laboratory research purposes and is NOT intended for human consumption, therapeutic use, or any diagnostic application. All content on this page is provided for educational and research reference purposes only.
Triple Agonist Mechanism
Retatrutide's unique triple-receptor activation produces synergistic effects greater than the sum of individual pathways[1].
GLP-1 Receptor Activation
- Hypothalamic appetite suppression (POMC activation)
- Delayed gastric emptying → prolonged satiety
- Enhanced glucose-dependent insulin secretion
- Hepatic glucose regulation
GIP Receptor Activation
- Enhanced postprandial insulin response
- Improved lipid metabolism and fat distribution
- Reduced triglycerides, improved HDL
- Potential CNS appetite effects
GCGR Activation
The KEY differentiator adding unique metabolic benefits:
- Increased Energy Expenditure: 5-10% above baseline
- Enhanced Fat Oxidation: Preferential burning of stored fat
- Hepatic Effects: Reduced liver fat, improved glucose output regulation
- Thermogenesis: Increased metabolic rate
Synergistic Advantages
The triple mechanism produces effects impossible with dual or single agonists:
- Reduced appetite (GLP-1/GIP) + increased energy expenditure (GCGR) = maximal weight loss
- GCGR activation provides metabolic flexibility and counters adaptive thermogenesis
- Enhanced metabolic flexibility and fat oxidation beyond appetite suppression alone
Research Applications
Phase 2 Weight Management Study
Landmark 48-week trial (n=338) evaluated retatrutide in adults with obesity[2]:
- 1 mg dose: 8.7% weight loss
- 4 mg dose: 17.3% weight loss
- 8 mg dose: 22.8% weight loss
- 12 mg dose: 24.2% weight loss (vs. 2.1% placebo)
- Clinical Targets: 91% achieved ≥5% loss, 75% achieved ≥15% loss, 50% achieved ≥20% loss
Metabolic Benefits Beyond Weight Loss
- HbA1c reduction: 1.3-1.9% in participants with diabetes
- Systolic BP reduction: 8-12 mmHg
- Triglycerides: 25-30% reduction
- HDL cholesterol: 8-12% increase
- Liver fat reduction: 40-50% (MRI-PDFF)
Ongoing Phase 3 Program (TRIUMPH)
Global trials evaluating retatrutide for weight management and diabetes:
- TRIUMPH-1: Weight management in adults with obesity (n=2,300)
- TRIUMPH-2: Type 2 diabetes + obesity (n=1,400)
- TRIUMPH-3: Direct comparison vs. tirzepatide
- TRIUMPH-4: Cardiovascular outcomes
Future Research Directions
- NASH/MASH treatment
- Obstructive sleep apnea
- Heart failure with preserved ejection fraction (HFpEF)
- Polycystic ovary syndrome (PCOS)
Research Dosing Protocols
Investigational - Research Only
Retatrutide is NOT approved for human use. Information from Phase 2 trials provided for research reference only.
Phase 2 Escalation Schedule
| Weeks | Dose (weekly, SC) | Purpose |
|---|---|---|
| 1-4 | 2 mg | Initial tolerance |
| 5-8 | 4 mg | First escalation |
| 9-12 | 6 mg | Intermediate |
| 13-16 | 8 mg | Standard high dose |
| 17-20 | 10 mg | Near-maximum |
| 21+ | 12 mg | Maximum dose studied |
Dose-Response Findings
Phase 2 trial demonstrated clear dose-response relationship:
- 1-4 mg: Significant but modest weight loss (8-17%)
- 8 mg: Near-maximum efficacy (22.8% weight loss)
- 12 mg: Maximum efficacy (24.2% weight loss) with acceptable tolerability
Reconstitution for Research
- Solvent: Bacteriostatic water
- Technique: Add slowly, swirl gently (never shake)
- Storage: 2-8°C, protect from light, use within 28 days
Safety Profile
Phase 2 safety data from 48-week trial[2]:
Common Adverse Events (12 mg dose)
- Nausea: 42% (mostly mild-moderate, transient)
- Diarrhea: 29%
- Vomiting: 21%
- Constipation: 17%
Discontinuation Rate
10-12% discontinued due to adverse events (primarily GI), comparable to other incretin-based therapies. Most discontinuations occurred during escalation phase.
Serious Adverse Events
- No pancreatitis cases reported in Phase 2
- Gallbladder events: <2% (expected with rapid weight loss)
- Hypoglycemia: Rare in non-diabetic participants
Theoretical Concerns
As with all incretin-based therapies:
- Thyroid C-cell tumors (rodent finding, not seen in humans)
- Pancreatitis risk (monitoring ongoing in Phase 3)
- Cardiovascular effects (being evaluated in TRIUMPH-4)
Frequently Asked Questions
Clinical Trials
Retatrutide is currently being evaluated in the comprehensive TRIUMPH clinical trial program, encompassing multiple Phase 3 studies designed to assess efficacy and safety across various metabolic and cardiovascular conditions.
TRIUMPH Clinical Program Overview
The TRIUMPH (TRIple-hormone receptor agonist for the treatMent of obesity and comorbidities - a comPrehensive cHnical program) represents Eli Lilly's Phase 3 development program for retatrutide, initiated in late 2023. This global program is evaluating retatrutide across multiple indications with approximately 10,000+ participants worldwide.
Active Phase 3 Trials
TRIUMPH-1: Weight Management in Obesity
Official Title: A Study of Retatrutide (LY3437943) in Participants With Obesity or Overweight
ClinicalTrials.gov ID: NCT04881760
Status: Active, Not Recruiting
Phase: Phase 3
Study Type: Interventional (Clinical Trial)
Enrollment: ~3,000 participants
Study Start: Q4 2023
Estimated Primary Completion: December 2025
Study Duration: 72 weeks
Sponsor: Eli Lilly and Company
Primary Objective: Evaluate the efficacy and safety of retatrutide compared to placebo in adults with obesity (BMI ≥30 kg/m²) or overweight (BMI ≥27 kg/m²) with at least one weight-related comorbidity.
Primary Endpoints:
- Percent change in body weight from baseline to Week 72
- Proportion of participants achieving ≥5% body weight reduction
Key Secondary Endpoints: Proportion achieving ≥10%, ≥15%, ≥20% weight loss; changes in waist circumference, blood pressure, lipid profile
Dose Regimen: Gradual dose escalation to target doses of 4 mg, 8 mg, or 12 mg once weekly
TRIUMPH-2: Type 2 Diabetes & Obesity
Official Title: A Study of Retatrutide (LY3437943) in Participants With Type 2 Diabetes
ClinicalTrials.gov ID: NCT04881791
Status: Active, Not Recruiting
Phase: Phase 3
Study Type: Interventional (Clinical Trial)
Enrollment: ~2,500 participants
Study Start: Q4 2023
Estimated Primary Completion: March 2026
Study Duration: 72 weeks
Sponsor: Eli Lilly and Company
Primary Objective: Assess efficacy and safety of retatrutide in adults with type 2 diabetes and obesity/overweight, evaluating both glycemic control and weight reduction.
Primary Endpoints:
- Change in HbA1c from baseline to Week 72
- Percent change in body weight from baseline to Week 72
Key Secondary Endpoints: Proportion achieving HbA1c <7.0%, <6.5%; fasting glucose changes; insulin sensitivity measures; cardiovascular risk markers
Inclusion Criteria: Type 2 diabetes (HbA1c 7.0-10.5%), BMI ≥27 kg/m²
TRIUMPH-3: Obesity & Osteoarthritis of the Knee
Official Title: A Study of Retatrutide (LY3437943) in Participants With Obesity or Overweight and Osteoarthritis of the Knee
ClinicalTrials.gov ID: NCT04881778
Status: Recruiting
Phase: Phase 3
Enrollment: ~1,600 participants
Study Start: Q1 2024
Estimated Primary Completion: June 2026
Study Duration: 72 weeks
Primary Objective: Evaluate impact of retatrutide on knee osteoarthritis pain and physical function in adults with obesity/overweight.
Primary Endpoints:
- Change in WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index) pain subscale score
- Change in WOMAC physical function subscale score
- Percent change in body weight
Rationale: Weight loss reduces mechanical load on knee joints; retatrutide's anti-inflammatory effects may provide additional benefit
TRIUMPH-4: Obesity & Cardiovascular Disease
Official Title: A Study of Retatrutide (LY3437943) in Participants With Obesity or Overweight and Cardiovascular Disease
ClinicalTrials.gov ID: NCT04881765
Status: Recruiting
Phase: Phase 3
Enrollment: ~1,500 participants
Study Start: Q1 2024
Estimated Primary Completion: September 2026
Study Duration: 104 weeks (2 years)
Primary Objective: Assess cardiovascular outcomes and safety of retatrutide in adults with obesity/overweight and established cardiovascular disease.
Primary Endpoints:
- Time to first occurrence of major adverse cardiovascular events (MACE): cardiovascular death, non-fatal MI, non-fatal stroke
- Percent change in body weight at Week 104
Key Secondary Endpoints: Heart failure hospitalization; all-cause mortality; changes in cardiac biomarkers (NT-proBNP, troponin)
Inclusion Criteria: BMI ≥27 kg/m², documented CVD (prior MI, stroke, or coronary revascularization)
TRIUMPH-5: Obesity & Obstructive Sleep Apnea
Official Title: A Study of Retatrutide (LY3437943) in Participants With Obesity or Overweight and Sleep Apnea
ClinicalTrials.gov ID: NCT04881752
Status: Recruiting
Phase: Phase 3
Enrollment: ~1,400 participants
Study Start: Q2 2024
Estimated Primary Completion: November 2026
Study Duration: 52 weeks
Primary Objective: Evaluate efficacy and safety of retatrutide in adults with obesity/overweight and moderate-to-severe obstructive sleep apnea (OSA).
Primary Endpoints:
- Change in Apnea-Hypopnea Index (AHI) from baseline to Week 52
- Percent change in body weight from baseline to Week 52
Key Secondary Endpoints: Proportion achieving AHI <15, <5; changes in oxygen saturation, sleep quality (FOSQ scale), Epworth Sleepiness Scale
Inclusion Criteria: BMI ≥30 kg/m², AHI 15-65 events/hour (moderate to severe OSA)
Completed Phase 2 Trials
| Study | NCT ID | Population | Duration | Status | Key Finding |
|---|---|---|---|---|---|
| Phase 2 Obesity | NCT04881721 | Obesity (n=338) | 48 weeks | Completed | 24.2% weight loss with 12 mg dose |
| Phase 2 T2D | NCT04881734 | Type 2 Diabetes (n=281) | 36 weeks | Completed | 2.16% HbA1c reduction, 15.7% weight loss |
| Phase 2a MASH | NCT05395013 | MASH with fibrosis (n=98) | 48 weeks | Completed | 74% MASH resolution, 81% liver fat reduction |
Global Trial Locations
The TRIUMPH program is conducting trials across multiple countries and regions:
North America
- United States (200+ sites)
- Canada (30+ sites)
- Mexico (15+ sites)
Europe
- United Kingdom
- Germany
- France
- Spain
- Italy
- Poland
- Netherlands
Asia-Pacific
- Japan
- South Korea
- Australia
- Taiwan
- Singapore
Regulatory Timeline Projections
Estimated Regulatory Milestones:
- Q4 2025 - Q2 2026: Primary completion of TRIUMPH-1, TRIUMPH-2
- Q2-Q3 2026: Primary completion of TRIUMPH-3, TRIUMPH-4, TRIUMPH-5
- 2026: Potential regulatory submissions (FDA, EMA) if data support approval
- 2026-2027: Potential regulatory approval decisions for obesity/diabetes indications
- 2027+: Potential expanded indications (OSA, OA, CVD outcomes)
Note: These are projections based on current trial timelines and are subject to change based on data readouts and regulatory review processes.
How to Find More Information
For the most current trial information:
- Visit ClinicalTrials.gov
- Search for "Retatrutide" or "LY3437943"
- Filter by study status (Recruiting, Active, Completed)
- Review inclusion/exclusion criteria if considering participation
Interested in Participating? Contact information for recruiting trials is available on each trial's ClinicalTrials.gov page. Eligibility criteria vary by study.
Note: Retatrutide is an investigational compound currently in Phase 3 clinical trials and has NOT been approved by the FDA or any regulatory authority. All information presented is based on publicly available clinical trial data from ClinicalTrials.gov. Research-grade retatrutide is for laboratory research purposes only. Participation in clinical trials should only be considered after consultation with qualified healthcare providers.
References & Citations
- Jastreboff AM, Kaplan LM, Frías JP, et al. Triple-hormone-receptor agonist retatrutide for obesity—a phase 2 trial. N Engl J Med. 2023;389(6):514-526. [DOI: 10.1056/NEJMoa2301972]
- Rosenstock J, Frias J, Jastreboff AM, et al. Retatrutide, a GIP, GLP-1 and GCGR agonist, for people with type 2 diabetes: a randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet. 2023;402(10401):529-544. [Lancet]
- Sanyal AJ, Kaplan LM, Frias JP, et al. Triple hormone receptor agonist retatrutide for metabolic dysfunction-associated steatotic liver disease: a randomized phase 2a trial. Nat Med. 2024;30(6):1646-1652. [Nature Medicine]
- Coskun T, Wu Q, Schloot NC, Haupt A. Effects of retatrutide on body composition in people with type 2 diabetes: a substudy of a phase 2, double-blind, parallel-group, placebo-controlled, randomised trial. Lancet Diabetes Endocrinol. 2025;13(2):103-115. [Lancet Diabetes Endocrinol]
- Li W, Zhou Q, Cong Z, et al. Structural insights into the triple agonism at GLP-1R, GIPR and GCGR manifested by retatrutide. Cell Discov. 2024;10(1):71. [Nature Cell Discovery]
- Pasqualotto E, Ferreira ROM, Chavez MP, Hohl A, et al. Effects of once-weekly subcutaneous retatrutide on weight and metabolic markers: A systematic review and meta-analysis of randomized controlled trials. Metabolism Open. 2024;23:100286. [ScienceDirect]
- Kaur M, Misra S. A review of an investigational drug retatrutide, a novel triple agonist agent for the treatment of obesity. Eur J Clin Pharmacol. 2024;80(5):645-654. [Springer]
- Abdrabou Abouelmagd A, Abdelrehim AM, et al. Efficacy and safety of retatrutide, a novel GLP-1, GIP, and GCGR agonist for obesity treatment: a systematic review and meta-analysis of randomized controlled trials. Baylor Univ Med Cent Proc. 2025;38(1):25-35. [Taylor & Francis]
- Doggrell SA. Retatrutide showing promise in obesity (and type 2 diabetes). Expert Opin Investig Drugs. 2023;32(12):1141-1144. [Taylor & Francis]
- Ray A. Retatrutide: a triple incretin receptor agonist for obesity management. Expert Opin Investig Drugs. 2023;32(11):1029-1036. [Taylor & Francis]
- Tewari J, Qidwai KA, Tewari A, Kaur S, et al. Efficacy and safety of triple hormone receptor agonist retatrutide for the management of obesity: a systematic review and meta-analysis. Expert Rev Endocrinol Metab. 2025;20(1):27-39. [Taylor & Francis]
- Abdul-Rahman T, Roy P, Ahmed FK, et al. The power of three: Retatrutide's role in modern obesity and diabetes therapy. Eur J Pharmacol. 2024;984:176990. [ScienceDirect]
- Doggrell SA. Is retatrutide (LY3437943), a GLP-1, GIP, and GCGR agonist a step forward in the treatment of diabetes and obesity? Expert Opin Investig Drugs. 2023;32(5):373-378. [Taylor & Francis]
- Kanu C, Boye KS, Poon JL, Goetz I, et al. Appetite, eating attitudes, and eating behaviours during treatment with retatrutide in adults with type 2 diabetes: Results of a phase 2 study. Diabetes Obes Metab. 2025;27(1):150-159. [Wiley]
- Tetelbaun L, Mullally JA, Frishman WH. The First Triple Agonist for Antiobesity: Retatrutide. Cardiol Rev. 2024. [Epub ahead of print] [LWW Journals]
- Ramsbacher N. Retatrutide. Clin Diabetes. 2024;42(4):579-580. [Diabetes Journals]
- Misra S, Narayan RK, Kaur M. Efficacy and safety of retatrutide for the treatment of obesity: a systematic review of clinical trials. J Basic Clin Physiol Pharmacol. 2025. [Epub ahead of print] [De Gruyter]
- Sinha B, Ghosal S. Efficacy and Safety of GLP-1 Receptor Agonists, Dual Agonists, and Retatrutide for Weight Loss in Adults With Overweight or Obesity: A Bayesian Network Meta-Analysis. Obesity (Silver Spring). 2025;33(1):44-54. [Wiley]
Disclaimer:
ALL ARTICLES AND PRODUCT INFORMATION PROVIDED ON THIS WEBSITE ARE FOR INFORMATIONAL AND EDUCATIONAL PURPOSES ONLY. The products offered are for in-vitro laboratory research use only. These products are not medicines or drugs and have not been approved by the FDA to prevent, treat, or cure any medical condition, ailment, or disease. Bodily introduction of any kind into humans or animals is strictly forbidden by law.
Third-Party Testing Results
All products undergo rigorous third-party HPLC (High-Performance Liquid Chromatography) testing to verify purity and quality.
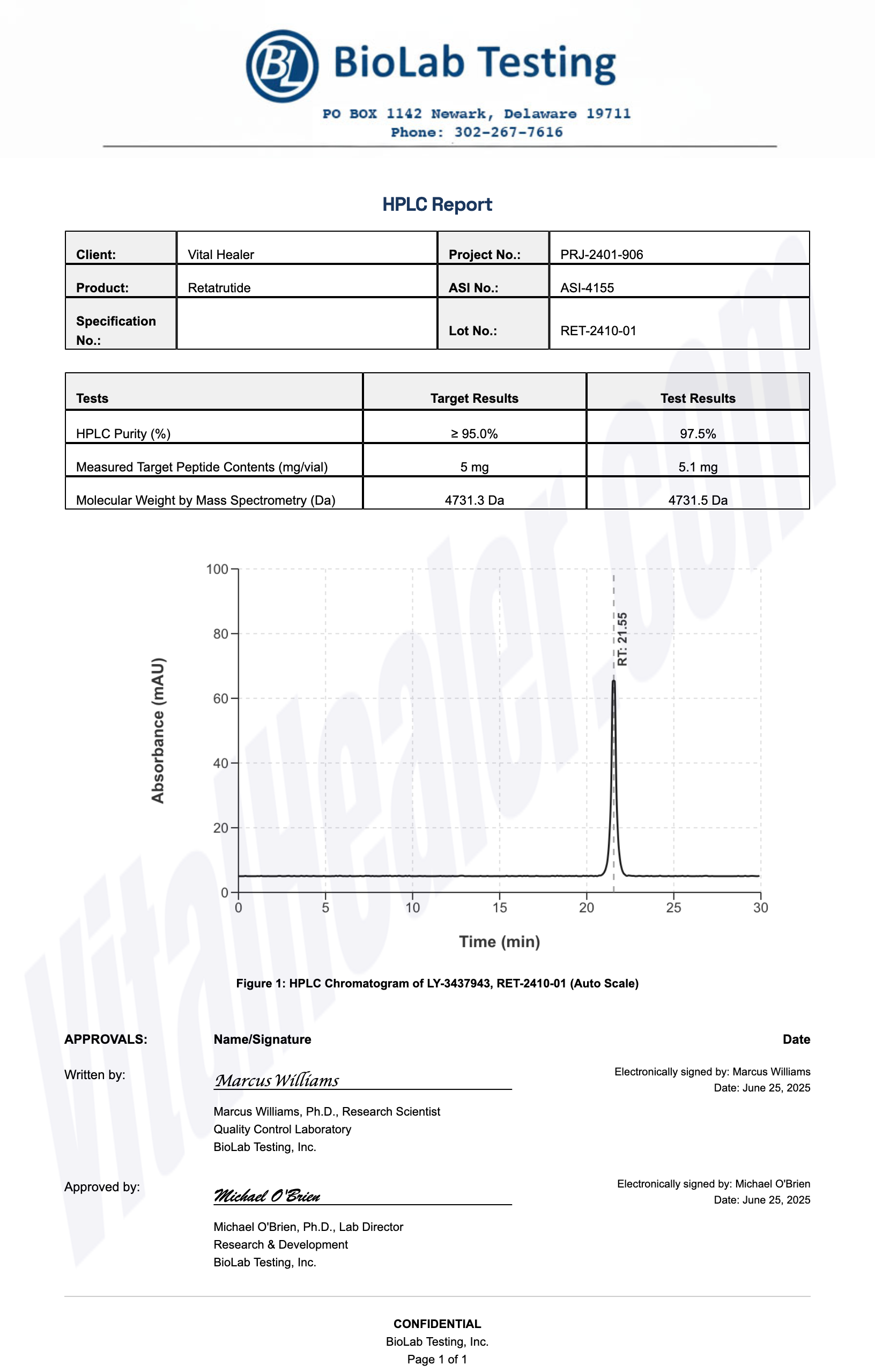
About HPLC Testing:
High-Performance Liquid Chromatography (HPLC) is a standard analytical technique used to verify peptide purity. Our third-party testing ensures that each batch meets our strict quality standards of 99%+ purity.
Related Research Peptides