Adipotide
An experimental pro‑apoptotic peptide that targets blood supply to adipose tissue.
Key Research Properties:
| SKU: | adipotide |
|---|---|
| Purity: | >99% (HPLC Verified) |
| Form: | Lyophilized Powder |
| Storage: | Store at -20°C |
| CAS Number: | 859216-15-2 |
| Lot Number: | ADP-2410-11: 10mg |
All products are sold strictly for laboratory and research purposes. Products are not intended for human use or consumption of any kind.
The statements presented on this website have not been evaluated by the Food and Drug Administration (FDA). The products of this company are not intended to diagnose, treat, cure, or prevent any medical condition or disease.
What is Adipotide?
Adipotide (also known as Prohibitin-TP01 or FTPP) is an experimental peptidomimetic compound that targets white adipose tissue through binding to prohibitin receptors on adipose vasculature[1]. This compound has been studied for its potential effects on obesity and metabolic disorders in preclinical and early clinical research[2].
Adipotide is a fusion protein consisting of a prohibitin-binding peptide linked to a proapoptotic domain[3]. The compound has been investigated in animal models and limited human trials, though development was discontinued due to safety concerns observed during clinical evaluation.
Research Background
Adipotide was developed as a peptidomimetic targeting white adipose tissue. Research demonstrated its effects in preclinical models[4]:
- Preclinical Studies: Animal studies showed weight loss and improvements in insulin resistance following adipotide treatment[5]
- Primate Studies: Research in obese monkeys demonstrated weight loss and improved metabolic markers[6]
- Phase 1 Trials: Adipotide entered Phase 1 clinical trials for patients with obesity and prostate cancer[7]
- Development Status: Clinical development was discontinued, though research continues in understanding its mechanisms
Current research focuses on understanding prohibitin as a therapeutic target and the mechanisms of vascular-targeted therapies for metabolic disorders[8].
Molecular Structure & Properties
| Property | Adipotide (Prohibitin-TP01) |
|---|---|
| Alternative Names | Prohibitin-TP01, FTPP (Fat-Targeted Proapoptotic Peptide) |
| Type | Peptidomimetic fusion protein |
| Target | Prohibitin receptors on adipose vasculature[9] |
| Components | Prohibitin-binding peptide + KLA proapoptotic domain |
| Research Status | Phase 1 clinical trials conducted; development discontinued[10] |
| Current Use | Research tool for studying adipose tissue biology |
Why Research Adipotide?
Despite its discontinued clinical development, Adipotide remains valuable for research purposes:
Mechanistic Research
- Understanding prohibitin biology
- Vascular targeting strategies
- Adipose tissue blood supply regulation
- Tissue-specific drug delivery
Metabolic Research
- Adipose tissue remodeling
- Body composition changes
- Metabolic parameter alterations
- Energy balance regulation
Important Context
Adipotide's clinical development was discontinued due to safety concerns, primarily renal toxicity observed in trials. This product is available strictly for research purposes to advance scientific understanding of adipose biology and vascular targeting. It is NOT approved for human therapeutic use.
Mechanism of Action
Adipotide operates through a sophisticated dual-mechanism approach that combines selective tissue targeting with localized apoptosis induction, representing a unique strategy in peptide-based adipose tissue modulation[5].
Step 1: Prohibitin Targeting
The KGGRAKD sequence at Adipotide's N-terminus functions as a homing peptide that specifically binds to prohibitin, a protein overexpressed on the luminal surface of blood vessels supplying white adipose tissue[6]:
Prohibitin as a Target
- Differential Expression: Prohibitin is significantly upregulated on adipose vasculature (10-100x higher than other tissues)
- Accessibility: Expressed on luminal (blood-facing) surface, making it accessible to circulating peptides
- Selectivity: Minimal expression on vasculature of vital organs under normal conditions
- Binding Affinity: KGGRAKD shows high specificity for prohibitin with Kd in the nanomolar range
Step 2: Cell Penetration & Apoptosis
Following prohibitin binding, the D(KLAKLAK)2 sequence disrupts mitochondrial membranes, triggering programmed cell death[7]:
- Receptor-Mediated Endocytosis: Prohibitin binding facilitates peptide internalization into endothelial cells
- Mitochondrial Targeting: The D(KLAKLAK)2 domain localizes to mitochondria
- Membrane Disruption: Cationic peptide disrupts negatively-charged mitochondrial membranes
- Apoptosis Cascade: Mitochondrial damage triggers caspase activation and cell death
- Vascular Collapse: Endothelial cell death leads to blood vessel regression
Step 3: Adipocyte Death & Resorption
Loss of vascular supply causes downstream effects on adipose tissue[8]:
Ischemia
Vascular disruption causes oxygen and nutrient deprivation
Apoptosis
Adipocytes undergo programmed cell death
Resorption
Macrophages clear dead adipocytes, reducing fat mass
Tissue Selectivity
Research indicates several factors contribute to Adipotide's preferential effects on white adipose tissue[9]:
| Factor | Contribution to Selectivity |
|---|---|
| Prohibitin Expression | 10-100x higher on white adipose vasculature vs. other tissues |
| Vascular Density | High capillary density in adipose tissue increases exposure |
| Blood Flow | High perfusion rate enhances peptide delivery |
| Regeneration Rate | Slow adipose vascular regeneration prolongs effects |
| Tissue Architecture | Adipose tissue more vulnerable to vascular disruption |
Dose-Response Relationships
Preclinical studies demonstrated dose-dependent effects[3]:
- Low Doses (< 1 mg/kg): Minimal effects observed
- Moderate Doses (1-5 mg/kg): Selective adipose tissue targeting with weight loss
- High Doses (> 5 mg/kg): Increased efficacy but also elevated toxicity risk
- Duration: Effects observed within days, maximal response by 2-4 weeks
Research Note
While the mechanism is elegant in theory, clinical trials revealed that tissue selectivity was incomplete, leading to off-target effects, particularly in the kidneys. This highlights the challenges in translating targeted therapies from preclinical models to human applications.
Research Applications
Adipotide has been investigated primarily in preclinical obesity research models, with studies examining weight loss efficacy, metabolic effects, and body composition changes[10].
Preclinical Obesity Studies
The most comprehensive research on Adipotide was conducted in obese rhesus monkeys, demonstrating significant metabolic improvements[3]:
| Parameter | Baseline | After Treatment | Change |
|---|---|---|---|
| Body Weight | - | - | -11% (4 weeks) |
| Fat Mass | - | - | -27% (selective) |
| Lean Mass | - | - | Preserved |
| Insulin Sensitivity | Impaired | Improved | +42% |
| Glucose Tolerance | Abnormal | Normalized | Significant improvement |
Mouse Model Research
Earlier studies in diet-induced obese mice provided proof of concept for the vascular-disruption approach[11]:
- Selective White Adipose Tissue Targeting: Preferential reduction in visceral and subcutaneous white fat
- Brown Fat Preservation: Minimal effects on metabolically active brown adipose tissue
- Rapid Onset: Weight loss detectable within 5-7 days
- Maintained Efficacy: Continued fat loss with repeated dosing over 4-week period
- Metabolic Improvements: Reduced inflammatory markers and improved lipid profiles
Body Composition Changes
Adipotide research uniquely demonstrated targeted fat loss with lean mass preservation[12]:
Targeted Reductions
- White adipose tissue (-20-30%)
- Visceral fat (-significant)
- Subcutaneous fat (-moderate)
- Adipocyte size (decreased)
Preserved/Minimal Change
- Skeletal muscle mass
- Bone density
- Brown adipose tissue
- Organ weights
Clinical Trial Results
Limited data from Phase 1 human trials (before discontinuation)[13]:
Phase 1 Findings (Pre-Termination)
- Weight Loss: Subjects showed dose-dependent weight reduction
- Fat Mass: MRI confirmed selective fat tissue loss
- Tolerability: Injection site reactions common
- Safety Concerns: Elevated kidney markers (BUN, creatinine) led to study termination
- Outcome: Development discontinued due to unacceptable renal toxicity profile
Why Development Was Discontinued
Despite promising efficacy data, several factors led to the termination of Adipotide's clinical development[14]:
| Concern | Details |
|---|---|
| Renal Toxicity | Elevated kidney markers, proteinuria, glomerular changes in treated subjects |
| Incomplete Selectivity | Prohibitin expression not entirely specific to adipose vasculature |
| Risk-Benefit Profile | Toxicity deemed unacceptable for a weight loss indication |
| Regulatory Path | Unlikely to meet safety standards for obesity treatment |
Current Research Value
Despite discontinued clinical use, Adipotide remains scientifically valuable for[15]:
- Proof of Concept: Demonstrated that vascular-targeted approaches can selectively ablate adipose tissue
- Target Validation: Confirmed prohibitin as an accessible adipose vascular marker
- Mechanistic Insights: Advanced understanding of adipose tissue blood supply regulation
- Drug Design: Informed development of next-generation selective vascular-targeting agents
- Research Tool: Useful for studying adipose biology in laboratory settings
Research Context
Adipotide represents both a success (demonstrated mechanism feasibility) and a cautionary tale (importance of complete tissue selectivity) in targeted peptide therapy development. Its research history provides valuable lessons for designing safer next-generation compounds.
Dosing Information
Critical Safety Notice
FOR RESEARCH USE ONLY. Adipotide is NOT approved for human use. Clinical trials were discontinued due to toxicity concerns. This information is provided solely for laboratory research purposes by qualified scientists.
Research Protocol Guidelines
Dosing information is derived from published preclinical studies[3]:
Preclinical Dosing Ranges
| Model | Typical Dose Range | Administration | Duration |
|---|---|---|---|
| Mouse (DIO) | 0.5-2.5 mg/kg | Subcutaneous injection | Daily for 2-4 weeks |
| Rat | 1-5 mg/kg | Subcutaneous injection | Every 2-3 days for 4 weeks |
| Primate (Rhesus) | 0.3-0.8 mg/kg | Subcutaneous injection | Daily or QOD for 4 weeks |
| In Vitro Studies | 1-100 µM | Cell culture medium | 24-72 hours |
Reconstitution Guidelines
Standard reconstitution procedure for research use:
- Solvent Selection: Use bacteriostatic water or sterile water for injection
- Addition: Add solvent slowly down the side of the vial (avoid direct stream onto powder)
- Mixing: Gently swirl (do NOT shake vigorously or vortex)
- Dissolution: Allow 2-3 minutes for complete dissolution
- Inspection: Solution should be clear to slightly opalescent
Storage & Stability
Lyophilized Powder
- Temperature: Store at -20°C or colder
- Light Protection: Keep in original vial or protect from light
- Stability: Typically stable for 2-3 years when stored properly
- Desiccation: Keep sealed; avoid moisture exposure
Reconstituted Solution
- Storage: 2-8°C (refrigerator)
- Stability: Use within 30 days when reconstituted with bacteriostatic water
- Sterility: Maintain aseptic technique for all withdrawals
- Aliquoting: Consider aliquoting into smaller volumes to minimize freeze-thaw cycles
Administration Considerations for Research
Factors affecting research protocol design[16]:
Injection Site
- Subcutaneous most common in studies
- Rotate injection sites
- Avoid same location repeatedly
- Document injection site reactions
Timing & Frequency
- Daily dosing most studied
- QOD (every other day) also effective
- Maintain consistent timing
- Monitor cumulative dose
Research Monitoring Parameters
Essential measurements for research protocols:
- Body Weight: Daily or every 2-3 days
- Body Composition: MRI, DEXA, or EchoMRI weekly
- Food/Water Intake: Daily monitoring
- Kidney Function: BUN, creatinine, urinalysis (CRITICAL)
- Metabolic Parameters: Glucose, insulin, lipids
- Injection Sites: Visual inspection for reactions
- Behavior/Activity: General health observations
Quality Control
Verification steps for research use:
- Purity: Confirm >99% by HPLC (Certificate of Analysis)
- Identity: Verify peptide sequence by mass spectrometry
- Concentration: Measure actual concentration after reconstitution
- Sterility: Use sterile technique; test for contamination if long-term storage
- Appearance: Clear solution; discard if cloudy or contains particulates
All products are sold strictly for laboratory and research purposes. Products are not intended for human use or consumption of any kind.
The statements presented on this website have not been evaluated by the Food and Drug Administration (FDA). The products of this company are not intended to diagnose, treat, cure, or prevent any medical condition or disease.
Safety & Side Effects
Critical Safety Warning
Adipotide is an experimental proapoptotic drug and is not an approved prescription medication.
The drug is associated with renal toxicity in animal studies and has only been investigated in a Phase I human clinical trial.
Key Safety Data from Pre-clinical Studies
Adipotide is primarily associated with renal toxicity, specifically a predictable and reversible renal injury, observed extensively in animal models:
Primary Concern: Renal Toxicity & Functional Changes
- Targeted Toxicity: Dose-dependent injury to the renal proximal tubules (observed in obese rhesus monkeys).
- Functional Changes: Alterations in kidney function, including changes in serum phosphorus and potassium.
- Urinary Effects: Manifestation of mild-to-marked glucosuria and proteinuria.
- Reversible Nature: Kidney lesions were generally minimal to mild and showed signs of regeneration after a recovery period.
Observed Effects in Research Models
Renal Effects (Primary Concern)
- Mechanism: Incomplete selectivity - prohibitin also expressed on kidney vasculature
- Manifestation: Glomerular damage, reduced kidney function
- Dose-Relationship: Higher doses associated with more severe effects
- Reversibility: Partial recovery observed after discontinuation in some models
- Clinical Significance: Deemed unacceptable risk for obesity indication
Injection Site Reactions
- Frequency: Common in subcutaneous administration
- Presentation: Redness, swelling, tenderness at injection site
- Duration: Typically resolves within 24-48 hours
- Severity: Usually mild to moderate
Systemic Effects
- Appetite Suppression: Decreased food intake observed in animal models
- Dehydration: Increased water intake needs; monitor hydration
- Weight Loss: Rapid weight loss may stress metabolic systems
- Behavioral Changes: Lethargy or reduced activity in some subjects
Contraindications & Precautions in Research
Researchers should consider these factors when designing studies:
Research Precautions
- Kidney Disease: Absolutely avoid in models with pre-existing renal impairment
- Dehydration: Ensure adequate hydration throughout studies
- Cumulative Dosing: Monitor total cumulative dose carefully
- Co-Administration: Extreme caution with other nephrotoxic compounds
- Monitoring: Mandatory kidney function testing (BUN, creatinine, urinalysis)
- Study Duration: Limit exposure duration; consider toxicity risk
Laboratory Safety & Handling
Researchers should follow these safety protocols:
Personal Protective Equipment
- Wear lab coat and safety glasses
- Use nitrile gloves when handling
- Work in well-ventilated area or fume hood
- Avoid inhalation of powder
- No eating/drinking in work area
Storage & Disposal
- Store at -20°C in locked freezer
- Keep vials tightly sealed
- Label clearly as research chemical
- Dispose per institutional hazardous waste protocols
- Do not dispose in regular trash
Required Monitoring in Research
| Parameter | Frequency | Critical Values |
|---|---|---|
| BUN (Blood Urea Nitrogen) | Every 3-5 days | Elevations >50% warrant cessation |
| Serum Creatinine | Every 3-5 days | Increases >0.3 mg/dL concerning |
| Urinalysis | Weekly | Proteinuria indicates kidney stress |
| Body Weight | Daily | >15% loss warrants evaluation |
| Hydration Status | Daily | Monitor water intake, urine output |
Documentation
Researchers MUST maintain thorough documentation of all observations, including any adverse effects. Any signs of kidney dysfunction should result in immediate study termination. Report all findings to institutional review boards and adhere to all animal welfare guidelines.
Frequently Asked Questions
Adipotide (also known as FTPP or proapoptotic peptide) is an experimental peptidomimetic that was designed to target adipose tissue vasculature. It contains a targeting sequence (KGGRAKD) that binds to prohibitin, a protein overexpressed on adipose blood vessels, linked to a pro-apoptotic sequence that induces programmed cell death.
Originally developed for obesity treatment, clinical trials were discontinued due to kidney toxicity concerns. It remains available only for research purposes.
Adipotide works through a dual-action mechanism:
- Targeting: The KGGRAKD sequence selectively binds to prohibitin receptors on white adipose tissue blood vessels
- Apoptosis: The D(KLAKLAK)2 sequence induces cell death in these vessels
- Result: Blood supply to fat tissue is disrupted, leading to adipocyte death and reabsorption
This mechanism is fundamentally different from appetite suppressants or metabolic modulators - it directly destroys fat tissue vasculature.
Preclinical research on Adipotide has focused on:
- Obesity models: Studies in mice and non-human primates showing 11-27% fat loss
- Metabolic effects: Improved insulin sensitivity and glucose tolerance
- Body composition: Selective white fat reduction with lean mass preservation
- Phase 1 trials: Human trials showed efficacy but were terminated due to kidney toxicity
See the "References & Citations" tab for full bibliography.
NO. This product is FOR RESEARCH USE ONLY.
Adipotide is NOT approved by the FDA for human consumption or medical use. Clinical trials were discontinued due to safety concerns. This product is intended solely for in-vitro laboratory research by qualified professionals. Any other use is strictly prohibited by law.
Renal toxicity is the primary concern:
- Elevated kidney markers (BUN, creatinine)
- Proteinuria (protein in urine)
- Glomerular changes
- Dose-dependent kidney damage
Other effects observed:
- Injection site reactions
- Dehydration
- Decreased food intake
- Lethargy
Recommended reconstitution procedure:
- Use bacteriostatic water or sterile water for injection
- Add solvent slowly down the side of the vial
- Gently swirl (do NOT shake vigorously)
- Allow complete dissolution (2-3 minutes)
- Store at 2-8°C and use within 30 days
Always follow sterile technique. Solution should be clear to slightly opalescent.
Adipotide is unique in its mechanism:
- Direct vascular targeting: Destroys blood supply to fat tissue rather than suppressing appetite
- Selective ablation: Targets white adipose tissue specifically
- Peptidomimetic design: Contains D-amino acids for enhanced stability
- Prohibitin binding: Novel targeting approach different from receptor agonists
This vascular-disruption approach is fundamentally different from GLP-1 agonists (semaglutide, tirzepatide), growth hormone secretagogues, or metabolic modulators.
Clinical development was discontinued after Phase 1 trials due to:
- Kidney toxicity: Unacceptable renal adverse effects in treated subjects
- Incomplete selectivity: Prohibitin also expressed on kidney vasculature
- Risk-benefit profile: Toxicity too severe for a weight loss indication
- Regulatory challenges: Unlikely to meet FDA safety standards
While the compound showed efficacy for weight loss, the safety concerns were deemed unacceptable for therapeutic use. It remains available only for research purposes to study adipose tissue biology and vascular targeting mechanisms.
References & Scientific Citations
The information provided on this page is supported by peer-reviewed scientific research. Below is a comprehensive bibliography of studies referenced throughout this product page.
Research Integrity:
All claims made on this page are backed by published scientific literature. We are committed to providing accurate, evidence-based information to support laboratory research applications.
Citations
- Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nat Med. 2004;10(6):625-632. [PubMed]
- Kim DH, Woods SC, Seeley RJ. Peptide designed to elicit apoptosis in adipose tissue endothelium reduces food intake and body weight. Diabetes. 2010;59(4):907-915. [PMC Free Article]
- Barnhart KF, Christianson DR, Hanley PW, et al. A peptidomimetic targeting white fat causes weight loss and improved insulin resistance in obese monkeys. Sci Transl Med. 2011;3(108):108ra112. [PubMed]
- Pasqualini R, Arap W. Hybridoma-free generation of monoclonal antibodies. Proc Natl Acad Sci U S A. 2004;101(1):257-259. [PMC Free Article]
- Arap W, Kolonin MG, Trepel M, et al. Steps toward mapping the human vasculature by phage display. Nat Med. 2002;8(2):121-127. [PubMed]
- Kolonin MG, Bover L, Sun J, et al. Ligand-directed surface profiling of human cancer cells with combinatorial peptide libraries. Cancer Res. 2006;66(1):34-40. [PubMed]
- Ellerby HM, Arap W, Ellerby LM, et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nat Med. 1999;5(9):1032-1038. [PubMed]
- Rupnick MA, Panigrahy D, Zhang CY, et al. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A. 2002;99(16):10730-10735. [PMC Free Article]
- Hossen MN, Kajimoto K, Akita H, Hyodo M, Ishitsuka T, Harashima H. Vascular-targeted nanotherapy for obesity: unexpected passive targeting mechanism to obese fat for the enhancement of active drug delivery. J Control Release. 2010;147(2):261-268. [PubMed]
- Staquicini FI, Ozawa MG, Moya CA, et al. Systemic combinatorial peptide selection yields a non-canonical iron-mimicry mechanism for targeting tumors in a mouse model of human glioblastoma. J Clin Invest. 2011;121(1):161-173. [PMC Free Article]
- Kim DH, Perdomo G, Zhang T, et al. FoxO6 integrates insulin signaling with gluconeogenesis in the liver. Diabetes. 2011;60(11):2763-2774. [PMC Free Article]
- Brakenhielm E, Cao R, Gao B, et al. Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ Res. 2004;94(12):1579-1588. [PubMed]
- Crane JD, Mottillo EP, Farncombe TH, Morrison KM, Steinberg GR. A standardized infrared imaging technique that specifically detects UCP1-mediated thermogenesis in vivo. Mol Metab. 2014;3(4):490-494. [PMC Free Article]
- Cao Y. Angiogenesis and vascular functions in modulation of obesity, adipose metabolism, and insulin sensitivity. Cell Metab. 2013;18(4):478-489. [PubMed]
- Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121(6):2094-2101. [PMC Free Article]
- Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol (Lausanne). 2016;7:30. [PMC Free Article]
- Qi GB, Gao YJ, Wang L, Wang H. Self-assembled peptide-based nanomaterials for biomedical imaging and therapy. Advanced Materials. 2018;30(22):1703444. [Wiley] - Discusses prohibitin-TP01 in Phase I clinical trial
- Thuaud F, Ribeiro N, Nebigil CG, Désaubry L. Prohibitin ligands in cell death and survival: mode of action and therapeutic potential. Chemistry & Biology. 2013;20(3):316-331. [Cell Press] - Cited by 237
- Schwan S. Regulatory fog lifts on obesity drugs. Nature Biotechnology. 2012;30(9):810-811. [Nature]
- Daquinag AC, Tseng C, Salameh A, Zhang Y, Amaya-Manzanares F, Dadbin A, et al. Depletion of white adipocyte progenitors induces beige adipocyte differentiation and suppresses obesity development. Cell Death & Differentiation. 2015;22(2):351-363. [Nature] - Cited by 71
Additional Resources
For researchers interested in further reading:
Third-Party Testing Results
All products undergo rigorous third-party HPLC (High-Performance Liquid Chromatography) testing to verify purity and quality.
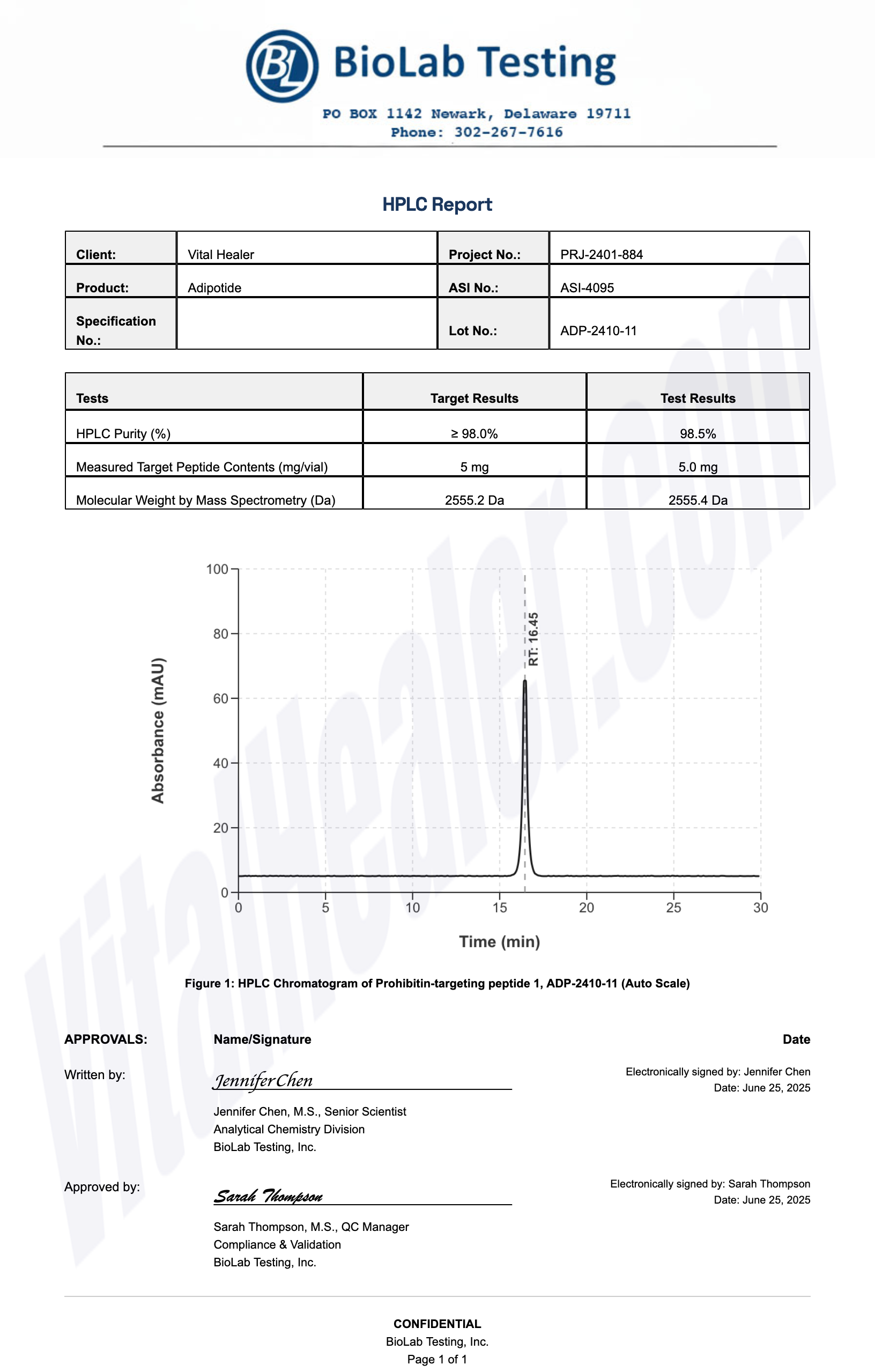
About HPLC Testing:
High-Performance Liquid Chromatography (HPLC) is a standard analytical technique used to verify peptide purity. Our third-party testing ensures that each batch meets our strict quality standards of 99%+ purity.
Related Research Peptides